Hypoxia: Signaling the Metastatic Cascade
- PMID: 28741527
- PMCID: PMC5808868
- DOI: 10.1016/j.trecan.2016.05.006
Hypoxia: Signaling the Metastatic Cascade
Abstract
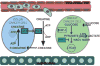
Hypoxia is a potent microenvironmental factor that promotes tumor metastasis. Recent studies have revealed mechanisms by which hypoxia and activation of hypoxia inducible factor (HIF)-dependent signaling promotes metastasis through the regulation of metabolic reprogramming, the stem cell phenotype, invasion, angiogenesis, immune suppression, the premetastatic niche, intravasation and/or extravasation, and resistance to apoptosis. These discoveries suggest novel paradigms in tumor metastasis and identify new opportunities for therapeutic intervention in the prevention and treatment of metastatic disease. Here, we review the impact of hypoxia and hypoxic signaling pathways in tumor and stromal cells on each step of the metastatic cascade.
Copyright © 2016. Published by Elsevier Inc.
Figures



Similar articles
-
Hypoxic control of metastasis.Science. 2016 Apr 8;352(6282):175-80. doi: 10.1126/science.aaf4405. Epub 2016 Apr 7. Science. 2016. PMID: 27124451 Free PMC article. Review.
-
Hypoxia-mediated metastasis.Adv Exp Med Biol. 2014;772:55-81. doi: 10.1007/978-1-4614-5915-6_3. Adv Exp Med Biol. 2014. PMID: 24272354 Review.
-
Hypoxia and the Tumor Secretome.Adv Exp Med Biol. 2019;1136:57-69. doi: 10.1007/978-3-030-12734-3_4. Adv Exp Med Biol. 2019. PMID: 31201716 Review.
-
Hypoxia and Metabolism in Metastasis.Adv Exp Med Biol. 2019;1136:87-95. doi: 10.1007/978-3-030-12734-3_6. Adv Exp Med Biol. 2019. PMID: 31201718 Review.
-
The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti-tumour strategies.Clin Exp Metastasis. 2018 Oct;35(7):563-599. doi: 10.1007/s10585-018-9930-x. Epub 2018 Aug 31. Clin Exp Metastasis. 2018. PMID: 30171389 Review.
Cited by
-
Hypoxia: Friend or Foe for drug delivery in Pancreatic Cancer.Cancer Lett. 2020 Nov 1;492:63-70. doi: 10.1016/j.canlet.2020.07.041. Epub 2020 Aug 18. Cancer Lett. 2020. PMID: 32822815 Free PMC article. Review.
-
Effects of Hyperoxia and Hyperoxic Oscillations on the Proteome of Murine Lung Microvascular Endothelium.Antioxidants (Basel). 2022 Nov 28;11(12):2349. doi: 10.3390/antiox11122349. Antioxidants (Basel). 2022. PMID: 36552557 Free PMC article.
-
Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches.Mol Carcinog. 2020 Mar;59(3):323-332. doi: 10.1002/mc.23157. Epub 2020 Jan 14. Mol Carcinog. 2020. PMID: 31943365 Free PMC article.
-
Hypoxic Signalling in Tumour Stroma.Front Oncol. 2018 May 29;8:189. doi: 10.3389/fonc.2018.00189. eCollection 2018. Front Oncol. 2018. PMID: 29896451 Free PMC article. Review.
-
Exploring the impact of circRNAs on cancer glycolysis: Insights into tumor progression and therapeutic strategies.Noncoding RNA Res. 2024 May 5;9(3):970-994. doi: 10.1016/j.ncrna.2024.05.001. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38770106 Free PMC article. Review.
References
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. - PubMed
-
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer research. 1998;58:1408–1416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

