Cloning, expression and purification of the α-carbonic anhydrase from the mantle of the Mediterranean mussel, Mytilus galloprovincialis
- PMID: 28741386
- PMCID: PMC6010101
- DOI: 10.1080/14756366.2017.1353502
Cloning, expression and purification of the α-carbonic anhydrase from the mantle of the Mediterranean mussel, Mytilus galloprovincialis
Abstract
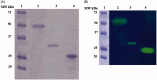
We cloned, expressed, purified, and determined the kinetic constants of the recombinant α-carbonic anhydrase (rec-MgaCA) identified in the mantle tissue of the bivalve Mediterranean mussel, Mytilus galloprovincialis. In metazoans, the α-CA family is largely represented and plays a pivotal role in the deposition of calcium carbonate biominerals. Our results demonstrated that rec-MgaCA was a monomer with an apparent molecular weight of about 32 kDa. Moreover, the determined kinetic parameters for the CO2 hydration reaction were kcat = 4.2 × 105 s-1 and kcat/Km of 3.5 × 107 M-1 ×s-1. Curiously, the rec-MgaCA showed a very similar kinetic and acetazolamide inhibition features when compared to those of the native enzyme (MgaCA), which has a molecular weight of 50 kDa. Analysing the SDS-PAGE, the protonography, and the kinetic analysis performed on the native and recombinant enzyme, we hypothesised that probably the native MgaCA is a multidomain protein with a single CA domain at the N-terminus of the protein. This hypothesis is corroborated by the existence in mollusks of multidomain proteins with a hydratase activity. Among these proteins, nacrein is an example of α-CA multidomain proteins characterised by a single CA domain at the N-terminus part of the entire protein.
Keywords: Carbonic anhydrase; bivalve; hydratase activity; metalloenzymes; multidomain protein; mussel; protonography; α-class enzyme.
Figures


Similar articles
-
Biochemical characterization of the native α-carbonic anhydrase purified from the mantle of the Mediterranean mussel, Mytilus galloprovincialis.J Enzyme Inhib Med Chem. 2017 Dec;32(1):632-639. doi: 10.1080/14756366.2017.1284069. J Enzyme Inhib Med Chem. 2017. PMID: 28229634 Free PMC article.
-
Evolution and diversity of alpha-carbonic anhydrases in the mantle of the Mediterranean mussel (Mytilus galloprovincialis).Sci Rep. 2019 Jul 18;9(1):10400. doi: 10.1038/s41598-019-46913-2. Sci Rep. 2019. PMID: 31320702 Free PMC article.
-
Identification two novel nacrein-like proteins involved in the shell formation of the Pacific oyster Crassostrea gigas.Mol Biol Rep. 2014 Jul;41(7):4273-8. doi: 10.1007/s11033-014-3298-z. Epub 2014 Mar 2. Mol Biol Rep. 2014. PMID: 24584662 Free PMC article.
-
Biochemical characterization of recombinant β-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis.J Enzyme Inhib Med Chem. 2015 Jun;30(3):366-70. doi: 10.3109/14756366.2014.931383. Epub 2014 Jul 17. J Enzyme Inhib Med Chem. 2015. PMID: 25032746
-
Cloning, expression, purification and sulfonamide inhibition profile of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum.Bioorg Med Chem Lett. 2016 Sep 1;26(17):4184-90. doi: 10.1016/j.bmcl.2016.07.060. Epub 2016 Jul 28. Bioorg Med Chem Lett. 2016. PMID: 27485387
Cited by
-
Activation Profile Analysis of CruCA4, an α-Carbonic Anhydrase Involved in Skeleton Formation of the Mediterranean Red Coral, Corallium rubrum.Molecules. 2017 Dec 28;23(1):66. doi: 10.3390/molecules23010066. Molecules. 2017. PMID: 29283417 Free PMC article.
-
Molecular Characterization of Carbonic Anhydrase II (CA II) and Its Potential Involvement in Regulating Shell Formation in the Pacific Abalone, Haliotis discus hannai.Front Mol Biosci. 2021 May 7;8:669235. doi: 10.3389/fmolb.2021.669235. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026840 Free PMC article.
-
The first activation study of a δ-carbonic anhydrase: TweCAδ from the diatom Thalassiosira weissflogii is effectively activated by amines and amino acids.J Enzyme Inhib Med Chem. 2018 Dec;33(1):680-685. doi: 10.1080/14756366.2018.1447570. J Enzyme Inhib Med Chem. 2018. PMID: 29536765 Free PMC article.
-
The first activation study of the β-carbonic anhydrases from the pathogenic bacteria Brucella suis and Francisella tularensis with amines and amino acids.J Enzyme Inhib Med Chem. 2019 Dec;34(1):1178-1185. doi: 10.1080/14756366.2019.1630617. J Enzyme Inhib Med Chem. 2019. PMID: 31282230 Free PMC article.
-
Carbonic Anhydrase in Pacific Abalone Haliotis discus hannai: Characterization, Expression, and Role in Biomineralization.Front Mol Biosci. 2021 Apr 15;8:655115. doi: 10.3389/fmolb.2021.655115. eCollection 2021. Front Mol Biosci. 2021. PMID: 33937335 Free PMC article.
References
-
- Supuran CT.Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. - PubMed
-
- Del Prete S, Vullo D, Fisher GM, et al. . Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum – the eta-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96. - PubMed
-
- Capasso C, Supuran CT.. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704. - PubMed
-
- Capasso C, Supuran CT.. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2015;22:2130–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
