DNA Oncogenic Virus-Induced Oxidative Stress, Genomic Damage, and Aberrant Epigenetic Alterations
- PMID: 28740569
- PMCID: PMC5504953
- DOI: 10.1155/2017/3179421
DNA Oncogenic Virus-Induced Oxidative Stress, Genomic Damage, and Aberrant Epigenetic Alterations
Abstract
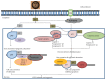
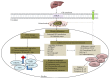
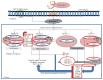
Approximately 20% of human cancers is attributable to DNA oncogenic viruses such as human papillomavirus (HPV), hepatitis B virus (HBV), and Epstein-Barr virus (EBV). Unrepaired DNA damage is the most common and overlapping feature of these DNA oncogenic viruses and a source of genomic instability and tumour development. Sustained DNA damage results from unceasing production of reactive oxygen species and activation of inflammasome cascades that trigger genomic changes and increased propensity of epigenetic alterations. Accumulation of epigenetic alterations may interfere with genome-wide cellular signalling machineries and promote malignant transformation leading to cancer development. Untangling and understanding the underlying mechanisms that promote these detrimental effects remain the major objectives for ongoing research and hope for effective virus-induced cancer therapy. Here, we review current literature with an emphasis on how DNA damage influences HPV, HVB, and EBV replication and epigenetic alterations that are associated with carcinogenesis.
Figures
Similar articles
-
Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis.Mutat Res. 2011 Jun 3;711(1-2):167-73. doi: 10.1016/j.mrfmmm.2011.02.015. Epub 2011 Mar 16. Mutat Res. 2011. PMID: 21419141 Review.
-
Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis.Int J Biol Sci. 2016 Oct 18;12(11):1309-1318. doi: 10.7150/ijbs.16564. eCollection 2016. Int J Biol Sci. 2016. PMID: 27877083 Free PMC article. Review.
-
Interplay between the DNA damage response and the life cycle of DNA tumor viruses.Tumour Virus Res. 2023 Dec;16:200272. doi: 10.1016/j.tvr.2023.200272. Epub 2023 Oct 31. Tumour Virus Res. 2023. PMID: 37918513 Free PMC article. Review.
-
Molecular biology of oncogenic inflammatory processes. I. Non-oncogenic and oncogenic pathogens, intrinsic inflammatory reactions without pathogens, and microRNA/DNA interactions (Review).Int J Oncol. 2012 Feb;40(2):305-49. doi: 10.3892/ijo.2011.1248. Epub 2011 Nov 4. Int J Oncol. 2012. PMID: 22076306 Review.
-
Interplay between DNA tumor viruses and the host DNA damage response.Curr Top Microbiol Immunol. 2013;371:229-57. doi: 10.1007/978-3-642-37765-5_9. Curr Top Microbiol Immunol. 2013. PMID: 23686238 Free PMC article. Review.
Cited by
-
How Oncogenic Viruses Exploit p62-Mediated Selective Autophagy for Cancer Development.Ann Immunol Immunother. 2021;3(1):134. Epub 2021 Mar 5. Ann Immunol Immunother. 2021. PMID: 34632457 Free PMC article. No abstract available.
-
Changes in oxidation-antioxidation function on the thymus of chickens infected with reticuloendotheliosis virus.BMC Vet Res. 2020 Dec 11;16(1):483. doi: 10.1186/s12917-020-02708-6. BMC Vet Res. 2020. PMID: 33308224 Free PMC article.
-
Antiviral Potential of Melissa officinalis L.: A Literature Review.Nutr Metab Insights. 2023 Jan 12;16:11786388221146683. doi: 10.1177/11786388221146683. eCollection 2023. Nutr Metab Insights. 2023. PMID: 36655201 Free PMC article. Review.
-
The master antioxidant defense is activated during EBV latent infection.J Virol. 2023 Nov 30;97(11):e0095323. doi: 10.1128/jvi.00953-23. Epub 2023 Oct 25. J Virol. 2023. PMID: 37877721 Free PMC article.
-
A multi-omic investigation of male lower urinary tract symptoms: Potential role for JC virus.PLoS One. 2021 Feb 25;16(2):e0246266. doi: 10.1371/journal.pone.0246266. eCollection 2021. PLoS One. 2021. PMID: 33630889 Free PMC article.
References
-
- Ziech D., Franco R., Pappa A., Panayiotidis M. I. Reactive oxygen species (ROS)—induced genetic and epigenetic alterations in human carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2011;711(1):167–173. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

