Amblyomma maculatum SECIS binding protein 2 and putative selenoprotein P are indispensable for pathogen replication and tick fecundity
- PMID: 28739494
- PMCID: PMC5583717
- DOI: 10.1016/j.ibmb.2017.07.006
Amblyomma maculatum SECIS binding protein 2 and putative selenoprotein P are indispensable for pathogen replication and tick fecundity
Abstract
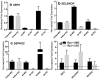
Selenium, a vital trace element, is incorporated into selenoproteins to produce selenocysteine. Our previous studies have revealed an adaptive co-evolutionary process that has enabled the spotted fever-causing tick-borne pathogen Rickettsia parkeri to survive by manipulating an antioxidant defense system associated with selenium, which includes a full set of selenoproteins and other antioxidants in ticks. Here, we conducted a systemic investigation of SECIS binding protein 2 (SBP2) and putative selenoprotein P (SELENOP) by transcript silencing in adult female Gulf-coast ticks (Amblyomma maculatum). Knockdown of the SBP2 and SELENOP genes depleted the respective transcript levels of these tick selenogenes, and caused differential regulation of other antioxidants. Importantly, the selenium level in the immature and mature tick stages increased significantly after a blood meal, but the selenium level decreased in ticks after the SBP2 and SELENOP knockdowns. Moreover, the SBP2 knockdown significantly impaired both transovarial transmission of R. parkeri to tick eggs and egg hatching. Overall, our data offer new insight into the relationship between the SBP2 selenoprotein synthesis gene and the putative tick SELENOP gene. It also augments our understanding of selenoprotein synthesis, selenium maintenance and utilization, and bacterial colonization of a tick vector.
Keywords: Amblyomma maculatum; RNA interference; Rickettsia parkeri; Selenium; Selenoproteins.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector.Microbiome. 2018 Aug 13;6(1):141. doi: 10.1186/s40168-018-0524-2. Microbiome. 2018. PMID: 30103809 Free PMC article.
-
Catalase is a determinant of the colonization and transovarial transmission of Rickettsia parkeri in the Gulf Coast tick Amblyomma maculatum.Insect Mol Biol. 2017 Aug;26(4):414-419. doi: 10.1111/imb.12304. Epub 2017 Apr 1. Insect Mol Biol. 2017. PMID: 28370634 Free PMC article.
-
Rickettsia parkeri colonization in Amblyomma maculatum: the role of superoxide dismutases.Parasit Vectors. 2016 May 20;9(1):291. doi: 10.1186/s13071-016-1579-1. Parasit Vectors. 2016. PMID: 27206371 Free PMC article.
-
Recent advances in understanding tick and rickettsiae interactions.Parasite Immunol. 2021 May;43(5):e12830. doi: 10.1111/pim.12830. Epub 2021 Apr 15. Parasite Immunol. 2021. PMID: 33713348 Free PMC article. Review.
-
From selenium to selenoproteins: synthesis, identity, and their role in human health.Antioxid Redox Signal. 2007 Jul;9(7):775-806. doi: 10.1089/ars.2007.1528. Antioxid Redox Signal. 2007. PMID: 17508906 Review.
Cited by
-
Spotted Fever Group Rickettsia Infection and Transmission Dynamics in Amblyomma maculatum.Infect Immun. 2019 Mar 25;87(4):e00804-18. doi: 10.1128/IAI.00804-18. Print 2019 Apr. Infect Immun. 2019. PMID: 30642897 Free PMC article.
-
A Peroxiredoxin From the Haemaphysalis longicornis Tick Affects Langat Virus Replication in a Hamster Cell Line.Front Cell Infect Microbiol. 2020 Jan 28;10:7. doi: 10.3389/fcimb.2020.00007. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32047725 Free PMC article.
-
The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector.Microbiome. 2018 Aug 13;6(1):141. doi: 10.1186/s40168-018-0524-2. Microbiome. 2018. PMID: 30103809 Free PMC article.
-
Cationic Glycopolyelectrolytes for RNA Interference in Tick Cells.Biomacromolecules. 2022 Jan 10;23(1):34-46. doi: 10.1021/acs.biomac.1c00824. Epub 2021 Nov 18. Biomacromolecules. 2022. PMID: 34793129 Free PMC article.
-
Is selenoprotein K required for Borrelia burgdorferi infection within the tick vector Ixodes scapularis?Parasit Vectors. 2019 Jun 7;12(1):289. doi: 10.1186/s13071-019-3548-y. Parasit Vectors. 2019. PMID: 31174589 Free PMC article.
References
-
- Adamson SW, Browning RE, Budachetri K, Ribeiro JMC, Karim S. Knockdown of selenocysteine-specific elongation factor in Amblyomma mac-ulatum alters the pathogen burden of Rickettsia parkeri with epigenetic control by the Sin3 histone deacetylase corepressor complex. PLoS One. 2013;8:e82012. http://dx.doi.org/10.1371/journal.pone.0082012. - DOI - PMC - PubMed
-
- Adamson S, Browning R, Singh P, Nobles S, Villarreal A, Karim S. Transcriptional activation of antioxidants may compensate for selenoprotein deficiencies in Amblyomma maculatum (Acari: ixodidae) injected with selK- or selM-dsRNA. Insect Mol Biol. 2014;23:497–510. http://dx.doi.org/10.1111/imb.12098. - DOI - PMC - PubMed
-
- Browning R, Karim S. RNA interference-mediated depletion of N-ethyl-maleimide sensitive fusion protein and synaptosomal associated protein of 25 kDa results in the inhibition of blood feeding of the gulf coast tick, Amblyomma maculatum. Insect Mol Biol. 2013 http://dx.doi.org/10.1111/imb.12017. - DOI - PMC - PubMed
-
- Bubenik JL, Miniard AC, Driscoll DM. Characterization of the UGA-recoding and SECIS-binding Activities of SECIS-binding Protein 2. 2015 http://dx.doi.org/10.1080/15476286.2014.996472. - DOI - PMC - PubMed
-
- Budachetri K, Karim S. An insight into the functional role of thioredoxin reductase, a selenoprotein, in maintaining normal native microbiota in the Gulf Coast tick (Amblyomma maculatum) Insect Mol Biol. 2015;24:570–581. http://dx.doi.org/10.1111/imb.12184. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

