TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders
- PMID: 28738878
- PMCID: PMC5525270
- DOI: 10.1186/s12974-017-0918-2
TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders
Abstract
Background: Inflammation during brain development participates in the pathogenesis of early brain injury and cognitive dysfunctions. Prenatal ethanol exposure affects the developing brain and causes neural impairment, cognitive and behavioral effects, collectively known as fetal alcohol spectrum disorders (FASD). Our previous studies demonstrate that ethanol activates the innate immune response and TLR4 receptor and causes neuroinflammation, brain damage, and cognitive defects in the developmental brain stage of adolescents. We hypothesize that by activating the TLR4 response, maternal alcohol consumption during pregnancy triggers the release of cytokines and chemokines in both the maternal sera and brains of fetuses/offspring, which impairs brain ontogeny and causes cognitive dysfunction.
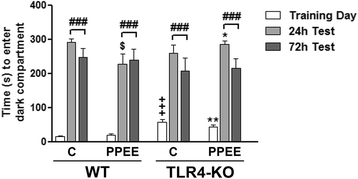
Methods: WT and TLR4-KO female mice treated with or without 10% ethanol in the drinking water during gestation and lactation were used. Cytokine/chemokine levels were determined by ELISA in the amniotic fluid, maternal serum, and cerebral cortex, as well as in the offspring cerebral cortex. Microglial and neuronal markers (evaluated by western blotting), myelin proteins (immunohistochemical and western blotting) and synaptic parameters (western blotting and electron microscopy) were assessed in the cortices of the WT and TLR4-KO pups on PND 0, 20, and 66. Behavioral tests (elevated plus maze and passive avoidance) were performed in the WT and TLR4-KO mice on PND 66 exposed or not to ethanol.
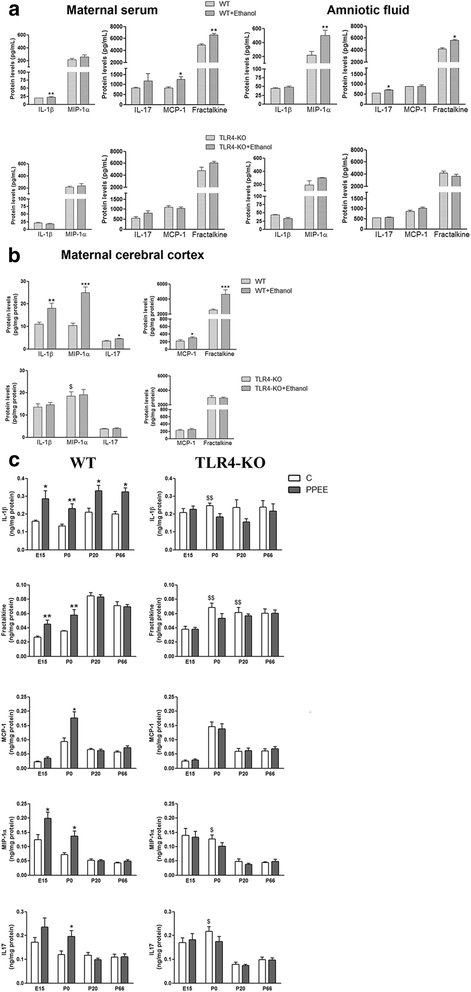
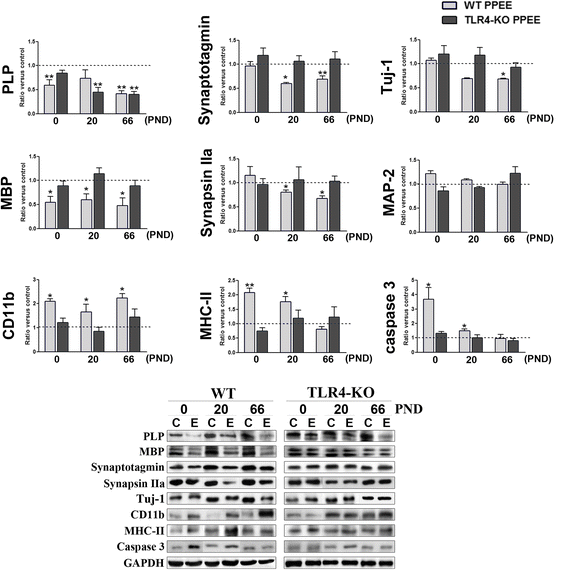
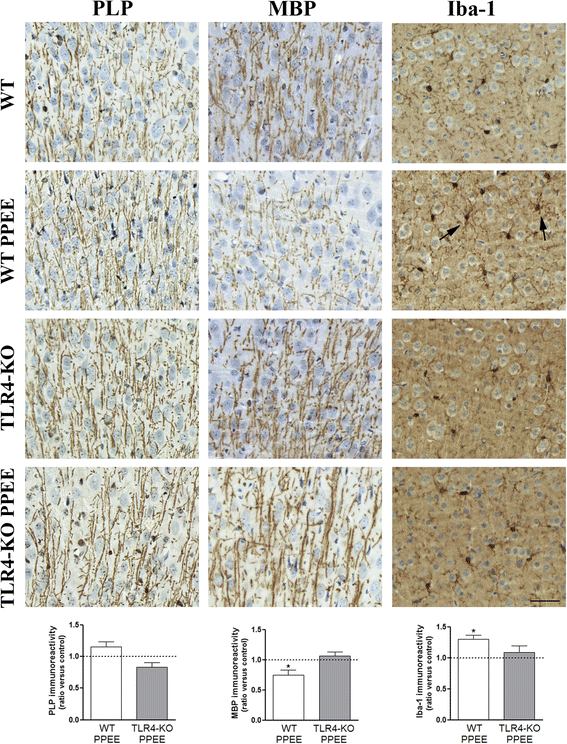
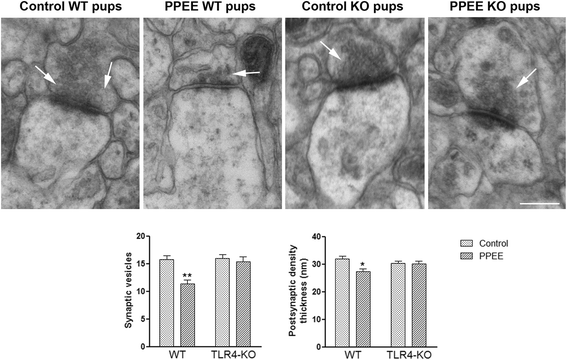
Results: We show that alcohol intake during gestation and lactation increases the levels of several cytokines/chemokines (IL-1β, IL-17, MIP-1α, and fractalkine) in the maternal sera, amniotic fluid, and brains of fetuses and offspring. The upregulation of cytokines/chemokines is associated with an increase in activated microglia markers (CD11b and MHC-II), and with a reduction in some synaptic (synaptotagmin, synapsin IIa) and myelin (MBP, PLP) proteins in the brains of offspring on days 0, 20, and 66 (long-term effects). These changes are associated with long-term behavioral impairments, in the 66-day-old alcohol-exposed pups. TLR4-deficient mice are protected against ethanol-induced cytokine/chemokine production in alcohol-treated dams and offspring, along with synaptic and myelin alterations, and the log-term behavioral dysfunction induced by ethanol in offspring.
Conclusions: These results suggest that the immune system activation, through the TLR4 response, might play an important role in the neurodevelopmental defects in FASD.
Keywords: Amniotic fluid; Behavior impairments; Cerebral cortex; Fetal alcohol spectrum disorders; Neuroinflammation; Prenatal ethanol exposure; Serum; TLR4.
Conflict of interest statement
Ethics approval
All the experimental procedures were approved by the Ethical Committee of Animal Experimentation of the Príncipe Felipe Research Center (Valencia, Spain) and were carried out in accordance with the guidelines approved by the European Communities Council Directive (2010/63/EU) and Spanish Royal Decree 1201/2005.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2.Neuropharmacology. 2015 Feb;89:352-9. doi: 10.1016/j.neuropharm.2014.10.014. Neuropharmacology. 2015. PMID: 25446779
-
TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment.Brain Behav Immun. 2015 Mar;45:233-44. doi: 10.1016/j.bbi.2014.11.015. Epub 2014 Dec 5. Brain Behav Immun. 2015. PMID: 25486089
-
Maternal alcohol binge drinking induces persistent neuroinflammation associated with myelin damage and behavioural dysfunctions in offspring mice.Neuropharmacology. 2017 Sep 1;123:368-384. doi: 10.1016/j.neuropharm.2017.05.034. Epub 2017 Jun 29. Neuropharmacology. 2017. PMID: 28669901
-
Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System.Alcohol Clin Exp Res. 2016 Nov;40(11):2260-2270. doi: 10.1111/acer.13208. Epub 2016 Sep 21. Alcohol Clin Exp Res. 2016. PMID: 27650785 Review.
-
Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain.Crit Rev Clin Lab Sci. 2011 Jan-Feb;48(1):19-47. doi: 10.3109/10408363.2011.580567. Crit Rev Clin Lab Sci. 2011. PMID: 21657944 Review.
Cited by
-
Updating an Overview of Teratology.Methods Mol Biol. 2024;2753:1-38. doi: 10.1007/978-1-0716-3625-1_1. Methods Mol Biol. 2024. PMID: 38285332
-
Cholinergic and Neuroimmune Signaling Interact to Impact Adult Hippocampal Neurogenesis and Alcohol Pathology Across Development.Front Pharmacol. 2022 Mar 2;13:849997. doi: 10.3389/fphar.2022.849997. eCollection 2022. Front Pharmacol. 2022. PMID: 35308225 Free PMC article. Review.
-
Sex-specific deficits in biochemical but not behavioral responses to delay fear conditioning in prenatal alcohol exposure mice.Neurobiol Learn Mem. 2018 Dec;156:1-16. doi: 10.1016/j.nlm.2018.10.002. Epub 2018 Oct 12. Neurobiol Learn Mem. 2018. PMID: 30316893 Free PMC article.
-
Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder: A Literature Review.Brain Sci. 2022 Jun 16;12(6):792. doi: 10.3390/brainsci12060792. Brain Sci. 2022. PMID: 35741677 Free PMC article. Review.
-
Prenatal alcohol-induced sex differences in immune, metabolic and neurobehavioral outcomes in adult rats.Brain Behav Immun. 2021 Nov;98:86-100. doi: 10.1016/j.bbi.2021.08.207. Epub 2021 Aug 11. Brain Behav Immun. 2021. PMID: 34390803 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

