Cladosporol A triggers apoptosis sensitivity by ROS-mediated autophagic flux in human breast cancer cells
- PMID: 28728544
- PMCID: PMC5520384
- DOI: 10.1186/s12860-017-0141-0
Cladosporol A triggers apoptosis sensitivity by ROS-mediated autophagic flux in human breast cancer cells
Erratum in
-
Erratum to: Cladosporol a triggers apoptosis sensitivity by ROS-mediated autophagic flux in human breast cancer cells.BMC Cell Biol. 2017 Aug 4;18(1):27. doi: 10.1186/s12860-017-0143-y. BMC Cell Biol. 2017. PMID: 28778152 Free PMC article. No abstract available.
Abstract
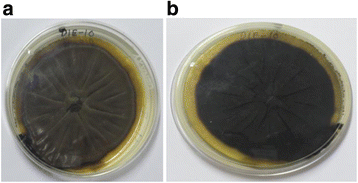
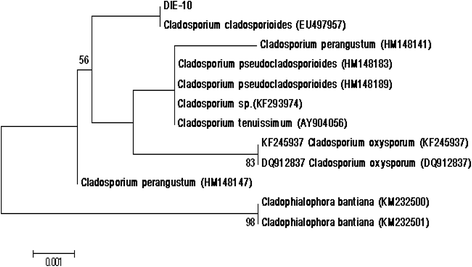
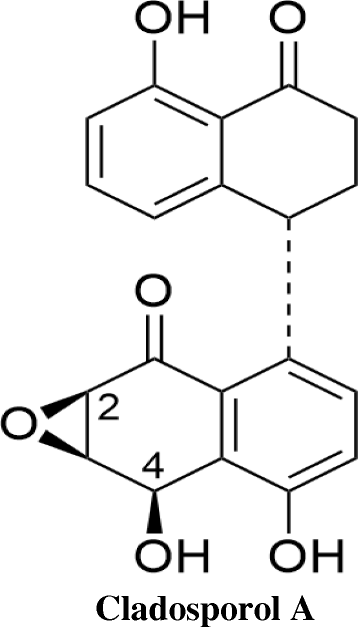
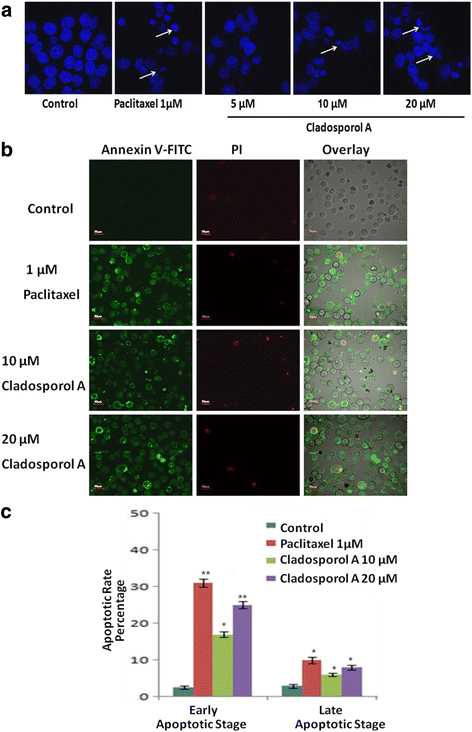
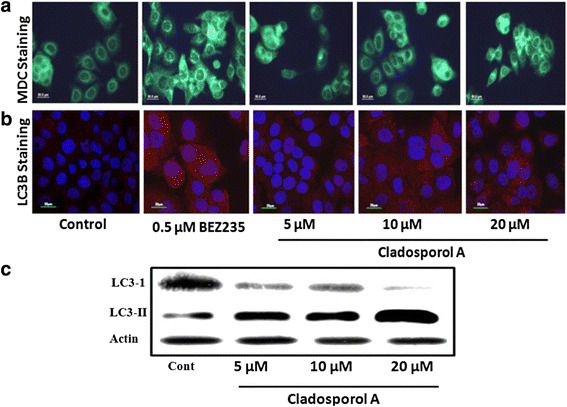
Background: Endophytes have proven to be an invaluable resource of chemically diverse secondary metabolites that act as excellent lead compounds for anticancer drug discovery. Here we report the promising cytotoxic effects of Cladosporol A (HPLC purified >98%) isolated from endophytic fungus Cladosporium cladosporioides collected from Datura innoxia. Cladosporol A was subjected to in vitro cytotoxicity assay against NCI60 panel of human cancer cells using MTT assay. We further investigated the molecular mechanism(s) of Cladosporol A induced cell death in human breast (MCF-7) cancer cells. Mechanistically early events of cell death were studied using DAPI, Annexin V-FITC staining assay. Furthermore, immunofluorescence studies were carried to see the involvement of intrinsic pathway leading to mitochondrial dysfunction, cytochrome c release, Bax/Bcl-2 regulation and flowcytometrically measured membrane potential loss of mitochondria in human breast (MCF-7) cancer cells after Cladosporol A treatment. The interplay between apoptosis and autophagy was studied by microtubule dynamics, expression of pro-apoptotic protein p21 and autophagic markers monodansylcadaverine staining and LC3b expression.
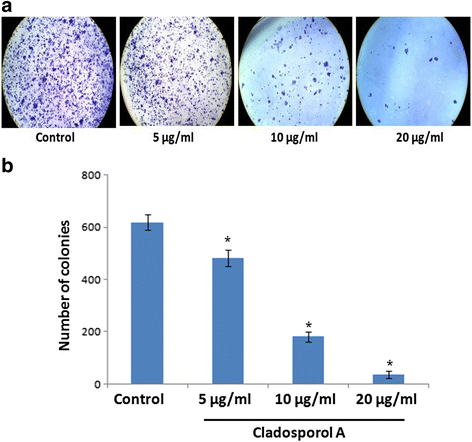
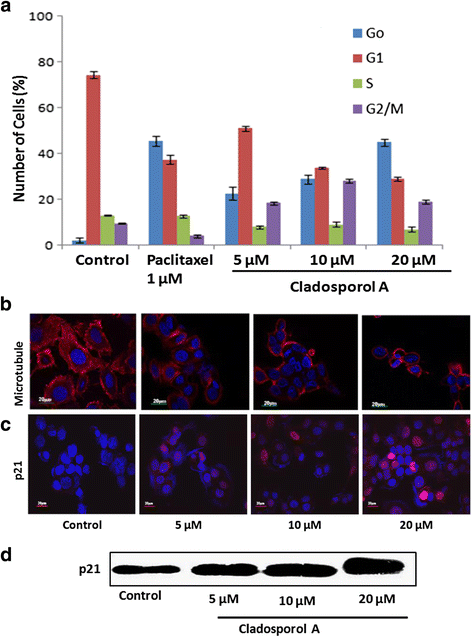
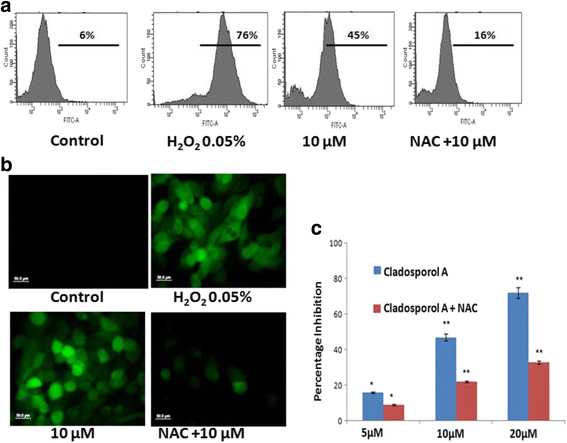
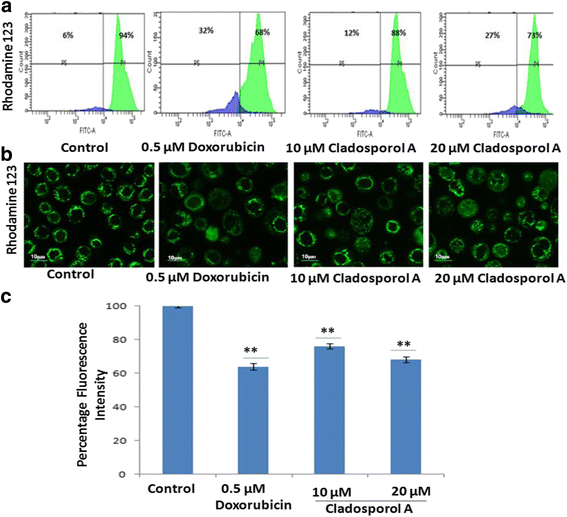
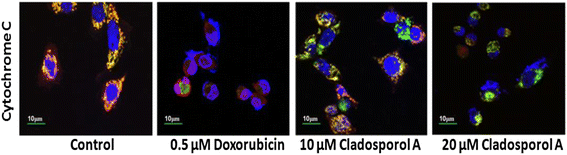
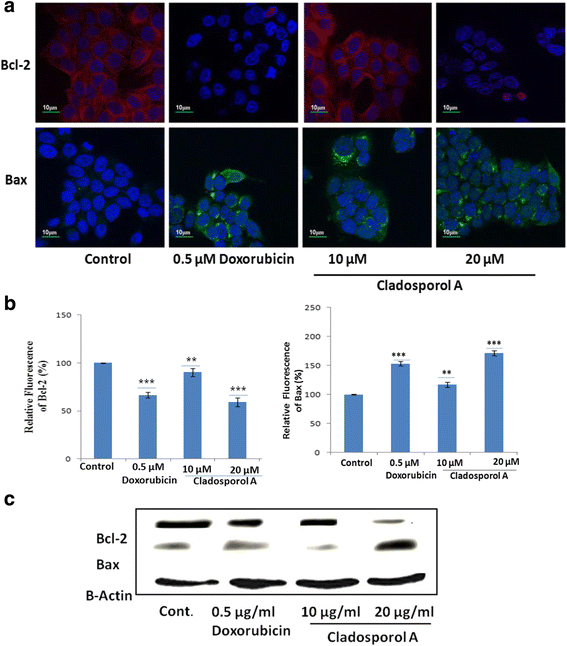
Results: Among NCI60 human cancer cell line panel Cladosporol A showed least IC50 value against human breast (MCF-7) cancer cells. The early events of apoptosis were characterized by phosphatidylserine exposure. It disrupts microtubule dynamics and also induces expression of pro-apoptotic protein p21. Moreover treatment of Cladosporol A significantly induced MMP loss, release of cytochrome c, Bcl-2 down regulation, Bax upregulation as well as increased monodansylcadaverine (MDC) staining and leads to LC3-I to LC3-II conversion.
Conclusion: Our experimental data suggests that Cladosporol A depolymerize microtubules, sensitize programmed cell death via ROS mediated autophagic flux leading to mitophagic cell death. The proposed mechanism of Cladosporol A -triggered apoptotic as well as autophagic death of human breast cancer (MCF-7) cells. The figure shows that Cladosporol A induced apoptosis through ROS mediated mitochondrial pathway and increased p21 protein expression in MCF-7 cells in vitro.
Keywords: Apoptosis; Breast cancer; Cladosporium cladosporioides; Cladosporol a; Endophytes; Reactive oxygen species.
Figures
Similar articles
-
Sirtinol, a class III HDAC inhibitor, induces apoptotic and autophagic cell death in MCF-7 human breast cancer cells.Int J Oncol. 2012 Sep;41(3):1101-9. doi: 10.3892/ijo.2012.1534. Epub 2012 Jun 26. Int J Oncol. 2012. PMID: 22751989
-
The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells.Drug Des Devel Ther. 2015 Mar 16;9:1627-52. doi: 10.2147/DDDT.S75378. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834401 Free PMC article.
-
Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells.Cancer Res. 2007 Aug 1;67(15):7406-20. doi: 10.1158/0008-5472.CAN-07-1089. Cancer Res. 2007. PMID: 17671211
-
Is mitochondrial generation of reactive oxygen species a trigger for autophagy?Autophagy. 2008 Feb;4(2):246-8. doi: 10.4161/auto.5432. Epub 2007 Dec 14. Autophagy. 2008. PMID: 18094624 Review.
-
Role of Reactive Oxygen Species in Estrogen Dependant Breast Cancer Complication.Anticancer Agents Med Chem. 2015;16(2):190-9. doi: 10.2174/1871520615666150518092315. Anticancer Agents Med Chem. 2015. PMID: 25980816 Review.
Cited by
-
Erratum to: Cladosporol a triggers apoptosis sensitivity by ROS-mediated autophagic flux in human breast cancer cells.BMC Cell Biol. 2017 Aug 4;18(1):27. doi: 10.1186/s12860-017-0143-y. BMC Cell Biol. 2017. PMID: 28778152 Free PMC article. No abstract available.
-
Differential Circulating Fungal Microbiome in Prostate Cancer Patients Compared to Healthy Control Individuals.J Immunol Res. 2022 Feb 3;2022:2574964. doi: 10.1155/2022/2574964. eCollection 2022. J Immunol Res. 2022. PMID: 35155686 Free PMC article.
-
PEGylated gold nanoparticles-ribonuclease induced oxidative stress and apoptosis in colorectal cancer cells.Bioimpacts. 2020;10(1):27-36. doi: 10.15171/bi.2020.04. Epub 2019 Jul 25. Bioimpacts. 2020. PMID: 31988854 Free PMC article.
-
Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential.Toxins (Basel). 2022 Feb 19;14(2):154. doi: 10.3390/toxins14020154. Toxins (Basel). 2022. PMID: 35202181 Free PMC article. Review.
-
Cytotoxic Mechanism of Sphaerodactylomelol, an Uncommon Bromoditerpene Isolated from Sphaerococcus coronopifolius.Molecules. 2021 Mar 4;26(5):1374. doi: 10.3390/molecules26051374. Molecules. 2021. PMID: 33806445 Free PMC article.
References
-
- Zhao J, Zhou L, Wang J, Shan T, Zhong L, Liu X, Gao X. Endophytic fungi for producing bioactive compounds originally from their host plants. Current Research, Technology and education Topics in applied Microbiology and Biotechnology. 2010;1:567–576.
-
- Koul M, Meena S, Kumar A, Sharma PR, Singamaneni V, Hassan SRU, Hamid A, Chaubey A, Prabhakar A, Gupta P, Singh S. Secondary metabolites from endophytic fungus Penicillium pinophilum induce ROS-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Med. 2016;82:344–355. doi: 10.1055/s-0035-1558308. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

