ALKBH7 drives a tissue and sex-specific necrotic cell death response following alkylation-induced damage
- PMID: 28726787
- PMCID: PMC5550884
- DOI: 10.1038/cddis.2017.343
ALKBH7 drives a tissue and sex-specific necrotic cell death response following alkylation-induced damage
Abstract
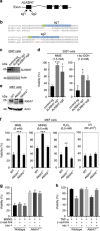
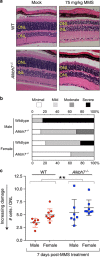
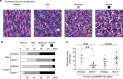
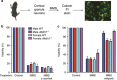
Regulated necrosis has emerged as a major cell death mechanism in response to different forms of physiological and pharmacological stress. The AlkB homolog 7 (ALKBH7) protein is required for regulated cellular necrosis in response to chemotherapeutic alkylating agents but its role within a whole organism is unknown. Here, we show that ALKBH7 modulates alkylation-induced cellular death through a tissue and sex-specific mechanism. At the whole-animal level, we find that ALKBH7 deficiency confers increased resistance to MMS-induced toxicity in male but not female mice. Moreover, ALKBH7-deficient mice exhibit protection against alkylation-mediated cytotoxicity in retinal photoreceptor and cerebellar granule cells, two cell types that undergo necrotic death through the initiation of the base excision repair pathway and hyperactivation of the PARP1/ARTD1 enzyme. Notably, the protection against alkylation-induced cerebellar degeneration is specific to ALKBH7-deficient male but not female mice. Our results uncover an in vivo role for ALKBH7 in mediating a sexually dimorphic tissue response to alkylation damage that could influence individual responses to chemotherapies based upon alkylating agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Human ALKBH7 is required for alkylation and oxidation-induced programmed necrosis.Genes Dev. 2013 May 15;27(10):1089-100. doi: 10.1101/gad.215533.113. Epub 2013 May 10. Genes Dev. 2013. PMID: 23666923 Free PMC article.
-
ALKBH7 mediates necrosis via rewiring of glyoxal metabolism.Elife. 2020 Aug 14;9:e58573. doi: 10.7554/eLife.58573. Elife. 2020. PMID: 32795389 Free PMC article.
-
Parp1 protects against Aag-dependent alkylation-induced nephrotoxicity in a sex-dependent manner.Oncotarget. 2016 Jul 19;7(29):44950-44965. doi: 10.18632/oncotarget.10440. Oncotarget. 2016. PMID: 27391435 Free PMC article.
-
MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents.DNA Repair (Amst). 2007 Aug 1;6(8):1079-99. doi: 10.1016/j.dnarep.2007.03.008. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17485253 Review.
-
Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration.Prog Retin Eye Res. 2005 Mar;24(2):275-306. doi: 10.1016/j.preteyeres.2004.08.002. Epub 2004 Nov 11. Prog Retin Eye Res. 2005. PMID: 15610977 Review.
Cited by
-
Hyaluronic acid is a negative regulator of mucosal fibroblast-mediated enhancement of HIV infection.Mucosal Immunol. 2021 Sep;14(5):1203-1213. doi: 10.1038/s41385-021-00409-3. Epub 2021 May 11. Mucosal Immunol. 2021. PMID: 33976386 Free PMC article.
-
PRMT5 regulates RNA m6A demethylation for doxorubicin sensitivity in breast cancer.Mol Ther. 2022 Jul 6;30(7):2603-2617. doi: 10.1016/j.ymthe.2022.03.003. Epub 2022 Mar 10. Mol Ther. 2022. PMID: 35278676 Free PMC article.
-
Rapid evolution of genes with anti-cancer functions during the origins of large bodies and cancer resistance in elephants.bioRxiv [Preprint]. 2024 Feb 29:2024.02.27.582135. doi: 10.1101/2024.02.27.582135. bioRxiv. 2024. PMID: 38463968 Free PMC article. Preprint.
-
Inflammation, necrosis, and the kinase RIP3 are key mediators of AAG-dependent alkylation-induced retinal degeneration.Sci Signal. 2019 Feb 12;12(568):eaau9216. doi: 10.1126/scisignal.aau9216. Sci Signal. 2019. PMID: 30755477 Free PMC article.
-
ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing.Nat Cell Biol. 2021 Jul;23(7):684-691. doi: 10.1038/s41556-021-00709-7. Epub 2021 Jul 12. Nat Cell Biol. 2021. PMID: 34253897 Free PMC article.
References
-
- Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 2014; 35: 24–32. - PubMed
-
- Newton K, Manning G. Necroptosis and inflammation. Annu Rev Biochem 2016. - PubMed
-
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015; 517: 311–320. - PubMed
-
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15: 135–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

