Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity
- PMID: 28725044
- PMCID: PMC6063081
- DOI: 10.1038/leu.2017.226
Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity
Abstract
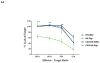
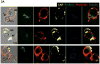
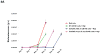
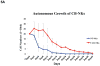
Chimeric antigen receptors (CARs) have been used to redirect the specificity of autologous T cells against leukemia and lymphoma with promising clinical results. Extending this approach to allogeneic T cells is problematic as they carry a significant risk of graft-versus-host disease (GVHD). Natural killer (NK) cells are highly cytotoxic effectors, killing their targets in a non-antigen-specific manner without causing GVHD. Cord blood (CB) offers an attractive, allogeneic, off-the-self source of NK cells for immunotherapy. We transduced CB-derived NK cells with a retroviral vector incorporating the genes for CAR-CD19, IL-15 and inducible caspase-9-based suicide gene (iC9), and demonstrated efficient killing of CD19-expressing cell lines and primary leukemia cells in vitro, with marked prolongation of survival in a xenograft Raji lymphoma murine model. Interleukin-15 (IL-15) production by the transduced CB-NK cells critically improved their function. Moreover, iC9/CAR.19/IL-15 CB-NK cells were readily eliminated upon pharmacologic activation of the iC9 suicide gene. In conclusion, we have developed a novel approach to immunotherapy using engineered CB-derived NK cells, which are easy to produce, exhibit striking efficacy and incorporate safety measures to limit toxicity. This approach should greatly improve the logistics of delivering this therapy to large numbers of patients, a major limitation to current CAR-T-cell therapies.
Conflict of interest statement
The authors have no conflict of interest.
Figures
















Comment in
-
Equipping NK Cells with CARs.Cancer Discov. 2017 Oct;7(10):OF2. doi: 10.1158/2159-8290.CD-NB2017-124. Epub 2017 Sep 6. Cancer Discov. 2017. PMID: 28877899
Similar articles
-
High Cytotoxic Efficiency of Lentivirally and Alpharetrovirally Engineered CD19-Specific Chimeric Antigen Receptor Natural Killer Cells Against Acute Lymphoblastic Leukemia.Front Immunol. 2020 Jan 24;10:3123. doi: 10.3389/fimmu.2019.03123. eCollection 2019. Front Immunol. 2020. PMID: 32117200 Free PMC article.
-
Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells.Sci Rep. 2019 Dec 10;9(1):18729. doi: 10.1038/s41598-019-55239-y. Sci Rep. 2019. PMID: 31822751 Free PMC article.
-
Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety.Leukemia. 2010 Jun;24(6):1160-70. doi: 10.1038/leu.2010.75. Epub 2010 Apr 29. Leukemia. 2010. PMID: 20428207 Free PMC article.
-
[Allogeneic CAR-NK cells: A promising alternative to autologous CAR-T cells - State of the art, sources of NK cells, limits and perspectives].Bull Cancer. 2021 Oct;108(10S):S81-S91. doi: 10.1016/j.bulcan.2021.06.007. Bull Cancer. 2021. PMID: 34920811 Review. French.
-
Cytotoxic function of umbilical cord blood natural killer cells: relevance to adoptive immunotherapy.Pediatr Hematol Oncol. 2011 Nov;28(8):640-6. doi: 10.3109/08880018.2011.613092. Epub 2011 Oct 4. Pediatr Hematol Oncol. 2011. PMID: 21970456 Review.
Cited by
-
CAR-NK cells for gastrointestinal cancer immunotherapy: from bench to bedside.Mol Cancer. 2024 Oct 23;23(1):237. doi: 10.1186/s12943-024-02151-3. Mol Cancer. 2024. PMID: 39443938 Free PMC article. Review.
-
A structural, genetic and clinical comparison of CAR-T cells and CAR-NK cells: companions or competitors?Front Immunol. 2024 Oct 4;15:1459818. doi: 10.3389/fimmu.2024.1459818. eCollection 2024. Front Immunol. 2024. PMID: 39430751 Free PMC article. Review.
-
Natural killer cell-based cancer immunotherapy: from basics to clinical trials.Exp Hematol Oncol. 2024 Oct 16;13(1):101. doi: 10.1186/s40164-024-00561-z. Exp Hematol Oncol. 2024. PMID: 39415291 Free PMC article. Review.
-
A Self-Activating IL-15 Chimeric Cytokine Receptor to Empower Cancer Immunotherapy.Immunotargets Ther. 2024 Oct 10;13:513-524. doi: 10.2147/ITT.S490498. eCollection 2024. Immunotargets Ther. 2024. PMID: 39403195 Free PMC article.
-
Antitumor effects of intracranial injection of B7-H3-targeted Car-T and Car-Nk cells in a patient-derived glioblastoma xenograft model.Cancer Immunol Immunother. 2024 Oct 5;73(12):256. doi: 10.1007/s00262-024-03808-0. Cancer Immunol Immunother. 2024. PMID: 39367952 Free PMC article.
References
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003 Jan;3(1):35–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

