rgs-CaM Detects and Counteracts Viral RNA Silencing Suppressors in Plant Immune Priming
- PMID: 28724770
- PMCID: PMC5599751
- DOI: 10.1128/JVI.00761-17
rgs-CaM Detects and Counteracts Viral RNA Silencing Suppressors in Plant Immune Priming
Abstract
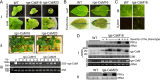
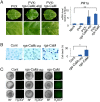
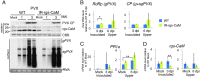
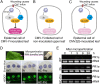
Primary infection of a plant with a pathogen that causes high accumulation of salicylic acid in the plant typically via a hypersensitive response confers enhanced resistance against secondary infection with a broad spectrum of pathogens, including viruses. This phenomenon is called systemic acquired resistance (SAR), which is a plant priming for adaption to repeated biotic stress. However, the molecular mechanisms of SAR-mediated enhanced inhibition, especially of virus infection, remain unclear. Here, we show that SAR against cucumber mosaic virus (CMV) in tobacco plants (Nicotiana tabacum) involves a calmodulin-like protein, rgs-CaM. We previously reported the antiviral function of rgs-CaM, which binds to and directs degradation of viral RNA silencing suppressors (RSSs), including CMV 2b, via autophagy. We found that rgs-CaM-mediated immunity is ineffective against CMV infection in normally growing tobacco plants but is activated as a result of SAR induction via salicylic acid signaling. We then analyzed the effect of overexpression of rgs-CaM on salicylic acid signaling. Overexpressed and ectopically expressed rgs-CaM induced defense reactions, including cell death, generation of reactive oxygen species, and salicylic acid signaling. Further analysis using a combination of the salicylic acid analogue benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) and the Ca2+ ionophore A23187 revealed that rgs-CaM functions as an immune receptor that induces salicylic acid signaling by simultaneously perceiving both viral RSS and Ca2+ influx as infection cues, implying its autoactivation. Thus, secondary infection of SAR-induced tobacco plants with CMV seems to be effectively inhibited through 2b recognition and degradation by rgs-CaM, leading to reinforcement of antiviral RNA silencing and other salicylic acid-mediated antiviral responses.IMPORTANCE Even without an acquired immune system like that in vertebrates, plants show enhanced whole-plant resistance against secondary infection with pathogens; this so-called systemic acquired resistance (SAR) has been known for more than half a century and continues to be extensively studied. SAR-induced plants strongly and rapidly express a number of antibiotics and pathogenesis-related proteins targeted against secondary infection, which can account for enhanced resistance against bacterial and fungal pathogens but are not thought to control viral infection. This study showed that enhanced resistance against cucumber mosaic virus is caused by a tobacco calmodulin-like protein, rgs-CaM, which detects and counteracts the major viral virulence factor (RNA silencing suppressor) after SAR induction. rgs-CaM-mediated SAR illustrates the growth versus defense trade-off in plants, as it targets the major virulence factor only under specific biotic stress conditions, thus avoiding the cost of constitutive activation while reducing the damage from virus infection.
Keywords: RNA interference; RNA silencing suppressor; calmodulin-like protein; cucumber mosaic virus; innate immunity; plant viruses; priming; salicylic acid signaling; systemic acquired resistance.
Copyright © 2017 American Society for Microbiology.
Figures




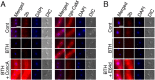
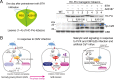
Similar articles
-
Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco.Plant J. 2006 Oct;48(2):217-27. doi: 10.1111/j.1365-313X.2006.02861.x. Plant J. 2006. PMID: 17018032
-
Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses.Proc Natl Acad Sci U S A. 1999 Jan 19;96(2):766-71. doi: 10.1073/pnas.96.2.766. Proc Natl Acad Sci U S A. 1999. PMID: 9892708 Free PMC article.
-
The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance.Mol Plant Microbe Interact. 2001 Jun;14(6):715-24. doi: 10.1094/MPMI.2001.14.6.715. Mol Plant Microbe Interact. 2001. PMID: 11386367
-
Signaling in induced resistance.Adv Virus Res. 2010;76:57-121. doi: 10.1016/S0065-3527(10)76003-6. Epub 2010 Mar 31. Adv Virus Res. 2010. PMID: 20965072 Review.
-
Local lesions and induced resistance.Adv Virus Res. 2009;75:73-117. doi: 10.1016/S0065-3527(09)07503-4. Epub 2010 Jan 13. Adv Virus Res. 2009. PMID: 20109664 Review.
Cited by
-
Calcium signaling: an emerging player in plant antiviral defense.J Exp Bot. 2024 Feb 28;75(5):1265-1273. doi: 10.1093/jxb/erad442. J Exp Bot. 2024. PMID: 37940194 Free PMC article.
-
Calcium Signalling in Plant Biotic Interactions.Int J Mol Sci. 2018 Feb 27;19(3):665. doi: 10.3390/ijms19030665. Int J Mol Sci. 2018. PMID: 29495448 Free PMC article. Review.
-
Molecular Regulation of Host Defense Responses Mediated by Biological Anti-TMV Agent Ningnanmycin.Viruses. 2019 Sep 3;11(9):815. doi: 10.3390/v11090815. Viruses. 2019. PMID: 31484426 Free PMC article.
-
CMV2b-Dependent Regulation of Host Defense Pathways in the Context of Viral Infection.Viruses. 2018 Nov 9;10(11):618. doi: 10.3390/v10110618. Viruses. 2018. PMID: 30423959 Free PMC article.
-
An Importin-β-like Protein from Nicotiana benthamiana Interacts with the RNA Silencing Suppressor P1b of the Cucumber Vein Yellowing Virus, Modulating Its Activity.Viruses. 2021 Nov 30;13(12):2406. doi: 10.3390/v13122406. Viruses. 2021. PMID: 34960675 Free PMC article.
References
-
- Chester KS. 1933. The problem of acquired physiological immunity in plants. Q Rev Biol 8:275–324. doi:10.1086/394440. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

