Evaluation of the Protective Effect of Deoxyribonucleic Acid Vaccines Encoding Granule Antigen 2 and 5 Against Acute Toxoplasmosis in BALB/c Mice
- PMID: 28719288
- PMCID: PMC5462584
- DOI: 10.4269/ajtmh.16-0548
Evaluation of the Protective Effect of Deoxyribonucleic Acid Vaccines Encoding Granule Antigen 2 and 5 Against Acute Toxoplasmosis in BALB/c Mice
Abstract
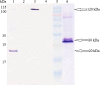
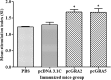
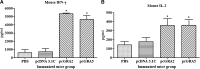
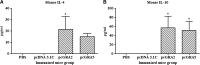
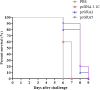
AbstractToxoplasma gondii infects a broad range of warm-blooded hosts, including humans. Important clinical manifestations include encephalitis in immunocompromised patients as well as miscarriage and fetal damage during early pregnancy. Toxoplasma gondii dense granule antigen 2 and 5 (GRA2 and GRA5) are essential for parasitophorous vacuole development of the parasite. To evaluate the potential of GRA2 and GRA5 as recombinant DNA vaccine candidates, these antigens were cloned into eukaryotic expression vector (pcDNA 3.1C) and evaluated in vaccination experiments. Recombinant DNA vaccines constructed with genes encoding GRAs were validated in Chinese hamster ovary cells before evaluation using lethal challenge of the virulent T. gondii RH strain in BALB/c mice. The DNA vaccines of pcGRA2 and pcGRA5 elicited cellular-mediated immune response with significantly higher levels of interferon-gamma, interleukin-2 (IL-2), IL-4, and IL-10 (P < 0.05) compared with controls. A mixed T-helper cell 1 (Th1)/Th2 response was associated with slightly prolonged survival. These findings provide evidence that DNA vaccination with GRA2 and GRA5 is associated with Th1-like cell-mediated immune responses. It will be worthwhile to construct recombinant multiantigen combining full-length GRA2 or/and GRA5 with various antigenic proteins such as the surface antigens and rhoptry antigens to improve vaccination efficacy.
Figures
Similar articles
-
Evaluation of protective immunity induced by DNA vaccination with genes encoding Toxoplasma gondii GRA17 and GRA23 against acute toxoplasmosis in mice.Exp Parasitol. 2017 Aug;179:20-27. doi: 10.1016/j.exppara.2017.06.002. Epub 2017 Jun 15. Exp Parasitol. 2017. PMID: 28625894
-
Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant.Vaccine. 2012 Feb 27;30(10):1800-6. doi: 10.1016/j.vaccine.2012.01.004. Epub 2012 Jan 10. Vaccine. 2012. PMID: 22240340
-
Protective efficacy induced by DNA prime and recombinant protein boost vaccination with Toxoplasma gondii GRA14 in mice.Microb Pathog. 2019 Sep;134:103601. doi: 10.1016/j.micpath.2019.103601. Epub 2019 Jun 15. Microb Pathog. 2019. PMID: 31212035
-
Vaccination against Toxoplasma gondii using rhoptry antigens: a systematic review.Comp Immunol Microbiol Infect Dis. 2018 Aug;59:32-40. doi: 10.1016/j.cimid.2018.09.005. Epub 2018 Sep 14. Comp Immunol Microbiol Infect Dis. 2018. PMID: 30290885 Review.
-
Veterinary vaccines against Toxoplasma gondii.Mem Inst Oswaldo Cruz. 2009 Mar;104(2):246-51. doi: 10.1590/s0074-02762009000200018. Mem Inst Oswaldo Cruz. 2009. PMID: 19430650 Review.
Cited by
-
[Toxoplasma gondii-Current drugs and future vaccines against an underestimated protozoan infection].Internist (Berl). 2021 Oct;62(10):1123-1132. doi: 10.1007/s00108-021-01155-1. Epub 2021 Aug 31. Internist (Berl). 2021. PMID: 34467425 Review. German.
-
A Review on the Prevalence of Toxoplasma gondii in Humans and Animals Reported in Malaysia from 2008-2018.Int J Environ Res Public Health. 2020 Jul 3;17(13):4809. doi: 10.3390/ijerph17134809. Int J Environ Res Public Health. 2020. PMID: 32635389 Free PMC article. Review.
-
REVIEW OF DNA VACCINE APPROACHES AGAINST THE PARASITE TOXOPLASMA GONDII.J Parasitol. 2021 Nov 1;107(6):882-903. doi: 10.1645/20-157. J Parasitol. 2021. PMID: 34852176 Free PMC article. Review.
-
Advances in Toxoplasma gondii Vaccines: Current Strategies and Challenges for Vaccine Development.Vaccines (Basel). 2021 Apr 21;9(5):413. doi: 10.3390/vaccines9050413. Vaccines (Basel). 2021. PMID: 33919060 Free PMC article. Review.
-
Toxoplasma gondii vaccine candidates: a concise review.Ir J Med Sci. 2023 Feb;192(1):231-261. doi: 10.1007/s11845-022-02998-9. Epub 2022 Apr 8. Ir J Med Sci. 2023. PMID: 35394635 Free PMC article. Review.
References
-
- Dubey JP. Toxoplasmosis of Animals and Humans. Boca Raton, FL: CRC Press; 2010. p. 313.
-
- Jackson MH, Hutchison WM. The prevalence and source of Toxoplasma infection in the environment. Adv Parasitol. 1989;28:55–105. - PubMed
-
- Bhopale GM. Development of a vaccine for toxoplasmosis: current status. Microbes Infect. 2003;5:457–462. - PubMed
-
- Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18:198–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

