The In Vivo Effects of the CB1-Positive Allosteric Modulator GAT229 on Intraocular Pressure in Ocular Normotensive and Hypertensive Mice
- PMID: 28719234
- PMCID: PMC5649393
- DOI: 10.1089/jop.2017.0037
The In Vivo Effects of the CB1-Positive Allosteric Modulator GAT229 on Intraocular Pressure in Ocular Normotensive and Hypertensive Mice
Abstract
Purpose: Orthosteric cannabinoid receptor 1 (CB1) activation leads to decreases in intraocular pressure (IOP). However, use of orthosteric CB1 agonists chronically has several disadvantages, limiting their usefulness as clinically relevant drugs. Allosteric modulators interact with topographically distinct sites to orthosteric ligands and may be useful to circumvent some of these disadvantages. The purpose of this study was to investigate the effects of the novel CB1-positive allosteric modulator (PAM) GAT229 on IOP.
Methods: IOP was measured using rebound tonometry in anesthetized normotensive C57Bl/6 mice and in a genetic model of ocular hypertension [nose, eyes, ears (nee) mice] before drug administration, and at 1, 6, and 12 h thereafter.
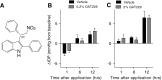
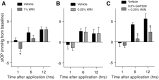
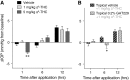
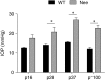
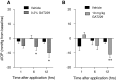
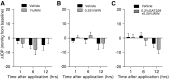
Results: In normotensive mice, topical administration of 5 μL GAT229 alone at either 0.2% or 2% did not reduce IOP. However, a subthreshold dose (0.25%) of the nonselective orthosteric CB1 agonist WIN 55,212-2, when combined with 0.2% GAT229, significantly reduced IOP compared with vehicle at 6 and 12 h. Similarly, combination of subthreshold Δ9-tetrahydrocannabinol (a nonselective orthosteric CB1 agonist; 1 mg/kg) with topical 0.2% GAT229 produced IOP lowering at 6 h. In nee mice, administration of topical 0.2% GAT229 or 10 mg/kg GAT229 alone was sufficient to lower IOP at 6 and 12 h, and 12 h, respectively.
Conclusions: The CB1 PAM GAT229 reduces IOP in ocular hypertensive mice and enhanced CB1-mediated IOP reduction when combined with subthreshold CB1 orthosteric ligands in normotensive mice. Administration of CB1 PAMs may provide a novel approach to reduce IOP with fewer of the disadvantages associated with orthosteric CB1 activation.
Keywords: allosteric modulator; cannabinoid receptor 1; cannabinoids; glaucoma; intraocular pressure.
Conflict of interest statement
M.E.M.K. is the founder and director of Panag Pharma Inc. Panag develops phytotherapeutics for local and regional treatment of pain and inflammation. E.A.C., A.M.S., P.M.K., R.G.P., G.A.T., and W.H.B. have no existing competing financial interests.
Figures
Similar articles
-
Topical WIN55212-2 alleviates intraocular hypertension in rats through a CB1 receptor mediated mechanism of action.J Ocul Pharmacol Ther. 2008 Feb;24(1):104-15. doi: 10.1089/jop.2007.0074. J Ocul Pharmacol Ther. 2008. PMID: 18201139 Free PMC article.
-
Design, synthesis, and pharmacological profiling of cannabinoid 1 receptor allosteric modulators: Preclinical efficacy of C2-group GAT211 congeners for reducing intraocular pressure.Bioorg Med Chem. 2021 Nov 15;50:116421. doi: 10.1016/j.bmc.2021.116421. Epub 2021 Sep 25. Bioorg Med Chem. 2021. PMID: 34634617
-
Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation.Molecules. 2020 Jan 20;25(2):417. doi: 10.3390/molecules25020417. Molecules. 2020. PMID: 31968549 Free PMC article.
-
Serotonin-2 receptor agonists as novel ocular hypotensive agents and their cellular and molecular mechanisms of action.Curr Drug Targets. 2010 Aug;11(8):978-93. doi: 10.2174/138945010791591278. Curr Drug Targets. 2010. PMID: 20426763 Review.
-
[Cannabinoid applications in glaucoma].Arch Soc Esp Oftalmol. 2011 Jan;86(1):16-23. doi: 10.1016/j.oftal.2010.11.015. Epub 2011 Feb 24. Arch Soc Esp Oftalmol. 2011. PMID: 21414525 Review. Spanish.
Cited by
-
Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine- Induced Dependence or Reward.Front Mol Neurosci. 2020 Apr 28;13:54. doi: 10.3389/fnmol.2020.00054. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32410959 Free PMC article.
-
Determination of the Cannabinoid CB1 Receptor's Positive Allosteric Modulator Binding Site through Mutagenesis Studies.Pharmaceuticals (Basel). 2024 Jan 24;17(2):154. doi: 10.3390/ph17020154. Pharmaceuticals (Basel). 2024. PMID: 38399369 Free PMC article.
-
CB1 Cannabinoid Receptor Signaling and Biased Signaling.Molecules. 2021 Sep 6;26(17):5413. doi: 10.3390/molecules26175413. Molecules. 2021. PMID: 34500853 Free PMC article. Review.
-
Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors.Br J Pharmacol. 2019 May;176(10):1455-1469. doi: 10.1111/bph.14440. Epub 2018 Aug 10. Br J Pharmacol. 2019. PMID: 29981240 Free PMC article.
-
Endocannabinoids at the synapse and beyond: implications for neuropsychiatric disease pathophysiology and treatment.Neuropsychopharmacology. 2023 Jan;48(1):37-53. doi: 10.1038/s41386-022-01438-7. Epub 2022 Sep 13. Neuropsychopharmacology. 2023. PMID: 36100658 Free PMC article. Review.
References
-
- King A., Azuara-Blanco A., and Tuulonen A. Glaucoma. BMJ. 346:f3518, 2013 - PubMed
-
- Heijl A., Leske M.C., Bengtsson B., et al. . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120:1268–1279, 2002 - PubMed
-
- Quigley H.A. Glaucoma. Lancet. 377:1367–1377, 2011 - PubMed
-
- Cairns E.A., Toguri J.T., Porter R.F., Szczesniak A.M., and Kelly M.E. Seeing over the horizon—targeting the endocannabinoid system for the treatment of ocular disease. J. Basic Clin. Physiol. Pharmacol. 27:253–265, 2016 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

