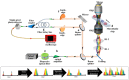
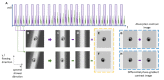
Microfluidic Imaging Flow Cytometry by Asymmetric-detection Time-stretch Optical Microscopy (ATOM)
- PMID: 28715367
- PMCID: PMC5608519
- DOI: 10.3791/55840
Microfluidic Imaging Flow Cytometry by Asymmetric-detection Time-stretch Optical Microscopy (ATOM)
Abstract
Scaling the number of measurable parameters, which allows for multidimensional data analysis and thus higher-confidence statistical results, has been the main trend in the advanced development of flow cytometry. Notably, adding high-resolution imaging capabilities allows for the complex morphological analysis of cellular/sub-cellular structures. This is not possible with standard flow cytometers. However, it is valuable for advancing our knowledge of cellular functions and can benefit life science research, clinical diagnostics, and environmental monitoring. Incorporating imaging capabilities into flow cytometry compromises the assay throughput, primarily due to the limitations on speed and sensitivity in the camera technologies. To overcome this speed or throughput challenge facing imaging flow cytometry while preserving the image quality, asymmetric-detection time-stretch optical microscopy (ATOM) has been demonstrated to enable high-contrast, single-cell imaging with sub-cellular resolution, at an imaging throughput as high as 100,000 cells/s. Based on the imaging concept of conventional time-stretch imaging, which relies on all-optical image encoding and retrieval through the use of ultrafast broadband laser pulses, ATOM further advances imaging performance by enhancing the image contrast of unlabeled/unstained cells. This is achieved by accessing the phase-gradient information of the cells, which is spectrally encoded into single-shot broadband pulses. Hence, ATOM is particularly advantageous in high-throughput measurements of single-cell morphology and texture - information indicative of cell types, states, and even functions. Ultimately, this could become a powerful imaging flow cytometry platform for the biophysical phenotyping of cells, complementing the current state-of-the-art biochemical-marker-based cellular assay. This work describes a protocol to establish the key modules of an ATOM system (from optical frontend to data processing and visualization backend), as well as the workflow of imaging flow cytometry based on ATOM, using human cells and micro-algae as the examples.
Similar articles
-
Asymmetric-detection time-stretch optical microscopy (ATOM) for ultrafast high-contrast cellular imaging in flow.Sci Rep. 2014 Jan 13;4:3656. doi: 10.1038/srep03656. Sci Rep. 2014. PMID: 24413677 Free PMC article.
-
Ultrafast Microfluidic Cellular Imaging by Optical Time-Stretch.Methods Mol Biol. 2016;1389:23-45. doi: 10.1007/978-1-4939-3302-0_3. Methods Mol Biol. 2016. PMID: 27460236
-
High-throughput imaging flow cytometry by optofluidic time-stretch microscopy.Nat Protoc. 2018 Jul;13(7):1603-1631. doi: 10.1038/s41596-018-0008-7. Nat Protoc. 2018. PMID: 29976951
-
Optofluidic time-stretch imaging - an emerging tool for high-throughput imaging flow cytometry.Lab Chip. 2016 May 10;16(10):1743-56. doi: 10.1039/c5lc01458a. Lab Chip. 2016. PMID: 27099993 Review.
-
High-throughput microfluidic imaging flow cytometry.Curr Opin Biotechnol. 2019 Feb;55:36-43. doi: 10.1016/j.copbio.2018.08.002. Epub 2018 Aug 15. Curr Opin Biotechnol. 2019. PMID: 30118968 Review.
Cited by
-
The Fusion of Microfluidics and Optics for On-Chip Detection and Characterization of Microalgae.Micromachines (Basel). 2021 Sep 22;12(10):1137. doi: 10.3390/mi12101137. Micromachines (Basel). 2021. PMID: 34683188 Free PMC article. Review.
References
-
- Lau AKS, Wong TTW, Shum HC, Wong KKY, Tsia KK. In: Imaging Flow Cytometry: Methods and Protocols. Barteneva NS, Vorobjev IA, editors. New York, NY: Springer New York; 2016. pp. 23–45.
-
- Goda K, Tsia KK, Jalali B. Serial time-encoded amplified imaging for real-time observation of fast dynamic phenomena. Nature. 2009;458(7242):1145–1149. - PubMed
-
- Goda K, Jalali B. Dispersive Fourier transformation for fast continuous single-shot measurements. Nat Photon. 2013;7(2):102–112.
-
- Lau AK, Shum HC, Wong KK, Tsia KK. Optofluidic time-stretch imaging - an emerging tool for high-throughput imaging flow cytometry. Lab Chip. 2016;16(10):1743–1756. - PubMed
-
- Lau AKS, Tang AHL, Xu J, Wong XW, Y KK, Tsia KK. Optical Time Stretch for High-Speed and High-Throughput Imaging - From Single-Cell to Tissue-Wide Scales. IEEE J. Sel. Top. in Quant. Electron. 2016;22(4)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources