Inhibition of WNT signaling attenuates self-renewal of SHH-subgroup medulloblastoma
- PMID: 28714964
- PMCID: PMC5680121
- DOI: 10.1038/onc.2017.232
Inhibition of WNT signaling attenuates self-renewal of SHH-subgroup medulloblastoma
Abstract
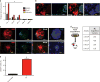
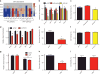
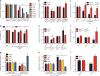
The SMOOTHENED inhibitor vismodegib is FDA approved for advanced basal cell carcinoma (BCC), and shows promise in clinical trials for SONIC HEDGEHOG (SHH)-subgroup medulloblastoma (MB) patients. Clinical experience with BCC patients shows that continuous exposure to vismodegib is necessary to prevent tumor recurrence, suggesting the existence of a vismodegib-resistant reservoir of tumor-propagating cells. We isolated such tumor-propagating cells from a mouse model of SHH-subgroup MB and grew them as sphere cultures. These cultures were enriched for the MB progenitor marker SOX2 and formed tumors in vivo. Moreover, while their ability to self-renew was resistant to SHH inhibitors, as has been previously suggested, this self-renewal was instead WNT-dependent. We show here that loss of Trp53 activates canonical WNT signaling in these SOX2-enriched cultures. Importantly, a small molecule WNT inhibitor was able to reduce the propagation and growth of SHH-subgroup MB in vivo, in an on-target manner, leading to increased survival. Our results imply that the tumor-propagating cells driving the growth of bulk SHH-dependent MB are themselves WNT dependent. Further, our data suggest combination therapy with WNT and SHH inhibitors as a therapeutic strategy in patients with SHH-subgroup MB, in order to decrease the tumor recurrence commonly observed in patients treated with vismodegib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis.Acta Neuropathol Commun. 2019 Jul 30;7(1):123. doi: 10.1186/s40478-019-0773-8. Acta Neuropathol Commun. 2019. PMID: 31362788 Free PMC article.
-
PI-3K Inhibitors Preferentially Target CD15+ Cancer Stem Cell Population in SHH Driven Medulloblastoma.PLoS One. 2016 Mar 3;11(3):e0150836. doi: 10.1371/journal.pone.0150836. eCollection 2016. PLoS One. 2016. PMID: 26938241 Free PMC article.
-
p53 Function Is Compromised by Inhibitor 2 of Phosphatase 2A in Sonic Hedgehog Medulloblastoma.Mol Cancer Res. 2019 Jan;17(1):186-198. doi: 10.1158/1541-7786.MCR-18-0485. Epub 2018 Sep 17. Mol Cancer Res. 2019. PMID: 30224541 Free PMC article.
-
SHH inhibitors for the treatment of medulloblastoma.Expert Rev Neurother. 2015;15(7):763-70. doi: 10.1586/14737175.2015.1052796. Epub 2015 May 31. Expert Rev Neurother. 2015. PMID: 26027634 Review.
-
Medulloblastoma, WNT-activated/SHH-activated: clinical impact of molecular analysis and histogenetic evaluation.Childs Nerv Syst. 2018 May;34(5):809-815. doi: 10.1007/s00381-018-3765-2. Epub 2018 Mar 26. Childs Nerv Syst. 2018. PMID: 29582169 Review.
Cited by
-
Wnt Signaling in Brain Tumors: A Challenging Therapeutic Target.Biology (Basel). 2023 May 16;12(5):729. doi: 10.3390/biology12050729. Biology (Basel). 2023. PMID: 37237541 Free PMC article. Review.
-
Noncanonical activation of GLI signaling in SOX2+ cells drives medulloblastoma relapse.Sci Adv. 2022 Jul 22;8(29):eabj9138. doi: 10.1126/sciadv.abj9138. Epub 2022 Jul 20. Sci Adv. 2022. PMID: 35857834 Free PMC article.
-
Differential Expression of Genes for Ubiquitin Ligases in Medulloblastoma Subtypes.Cerebellum. 2019 Jun;18(3):469-488. doi: 10.1007/s12311-019-1009-y. Cerebellum. 2019. PMID: 30810905
-
Dlp-mediated Hh and Wnt signaling interdependence is critical in the niche for germline stem cell progeny differentiation.Sci Adv. 2020 May 13;6(20):eaaz0480. doi: 10.1126/sciadv.aaz0480. eCollection 2020 May. Sci Adv. 2020. PMID: 32426496 Free PMC article.
-
Wnt Signaling in Thyroid Homeostasis and Carcinogenesis.Genes (Basel). 2018 Apr 10;9(4):204. doi: 10.3390/genes9040204. Genes (Basel). 2018. PMID: 29642644 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

