Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications
- PMID: 28714140
- PMCID: PMC5771986
- DOI: 10.1002/term.2512
Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications
Abstract
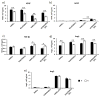
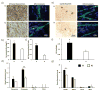
Self-sustainability after implantation is one of the critical obstacles facing large engineered tissues. A preformed functional vascular network provides an effective solution for solving the mass transportation problem. With the support of mural cells, endothelial cells (ECs) can form microvessels within engineered tissues. As an important mural cell, human mesenchymal stem cells (hMSCs) not only stabilize the engineered microvessel network, but also preserve their multi-potency when grown under optimal culture conditions. A prevascularized hMSC/extracellular matrix (ECM) sheet fabricated by the combination of hMSCs, ECs and a naturally derived nanofibrous ECM scaffold offers great opportunity for engineering mechanically strong and completely biological three-dimensional prevascularized tissues. The objective of this study was to create a prevascularized hMSC/ECM sheet by co-culturing ECs and hMSCs on a nanofibrous ECM scaffold. Physiologically low oxygen (2% O2 ) was introduced during the 7 day hMSC culture to preserve the stemness of hMSCs and thereby their capability to secrete angiogenic factors. The ECs were then included to form microvessels under normal oxygen (20% O2 ) for up to 7 days. The results showed that a branched and mature vascular network was formed in the co-culture condition. Angiogenic factors vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and angiopoietin-1 (Ang-1) were significantly increased by low-oxygen culture of hMSCs, which further stabilized and supported the maturation of microvessels. A differentiation assay of the prevascularized ECM scaffold demonstrated a retained hMSC multi-potency in the hypoxia cultured samples. The prevascularized hMSC/ECM sheet holds great promise for engineering three-dimensional prevascularized tissues for diverse applications.
Keywords: angiogenesis; extracellular matrix scaffold; mesenchymal stem cell; microvessel; nanofibrous scaffold; prevascularization.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures




Similar articles
-
Engineering stem cell cardiac patch with microvascular features representative of native myocardium.Theranostics. 2019 Apr 9;9(8):2143-2157. doi: 10.7150/thno.29552. eCollection 2019. Theranostics. 2019. PMID: 31149034 Free PMC article.
-
Preservation of microvascular integrity and immunomodulatory property of prevascularized human mesenchymal stem cell sheets.J Tissue Eng Regen Med. 2021 Mar;15(3):207-218. doi: 10.1002/term.3167. Epub 2021 Jan 28. J Tissue Eng Regen Med. 2021. PMID: 33432700
-
Hypoxia Created Human Mesenchymal Stem Cell Sheet for Prevascularized 3D Tissue Construction.Adv Healthc Mater. 2016 Feb 4;5(3):342-52. doi: 10.1002/adhm.201500744. Epub 2015 Dec 14. Adv Healthc Mater. 2016. PMID: 26663707
-
Preparation of three-dimensional vascularized MSC cell sheet constructs for tissue regeneration.Biomed Res Int. 2014;2014:301279. doi: 10.1155/2014/301279. Epub 2014 Jul 8. Biomed Res Int. 2014. PMID: 25110670 Free PMC article.
-
Osteogenic Differentiation Evaluation of an Engineered Extracellular Matrix Based Tissue Sheet for Potential Periosteum Replacement.ACS Appl Mater Interfaces. 2015 Oct 21;7(41):23239-47. doi: 10.1021/acsami.5b07386. Epub 2015 Oct 9. ACS Appl Mater Interfaces. 2015. PMID: 26419888
Cited by
-
Fibroblast-Generated Extracellular Matrix Guides Anastomosis during Wound Healing in an Engineered Lymphatic Skin Flap.Bioengineering (Basel). 2023 Jan 22;10(2):149. doi: 10.3390/bioengineering10020149. Bioengineering (Basel). 2023. PMID: 36829643 Free PMC article.
-
Pre-cultured, cell-encapsulating fibrin microbeads for the vascularization of ischemic tissues.J Biomed Mater Res A. 2024 Apr;112(4):549-561. doi: 10.1002/jbm.a.37580. Epub 2023 Jun 16. J Biomed Mater Res A. 2024. PMID: 37326361
-
Engineering stem cell cardiac patch with microvascular features representative of native myocardium.Theranostics. 2019 Apr 9;9(8):2143-2157. doi: 10.7150/thno.29552. eCollection 2019. Theranostics. 2019. PMID: 31149034 Free PMC article.
-
Mechanical strategies to promote vascularization for tissue engineering and regenerative medicine.Burns Trauma. 2024 Sep 30;12:tkae039. doi: 10.1093/burnst/tkae039. eCollection 2024. Burns Trauma. 2024. PMID: 39350780 Free PMC article. Review.
-
Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues.Cells. 2024 Oct 1;13(19):1638. doi: 10.3390/cells13191638. Cells. 2024. PMID: 39404401 Free PMC article. Review.
References
-
- Aguirre A, Planell JA, Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400(2):284–291. - PubMed
-
- Akhurst RJ, Lehnert SA, Faissner A, Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990;108(4):645–656. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–523. - PubMed
-
- Barati D, Shariati SRP, Moeinzadeh S, Melero-Martin JM, Khademhosseini A, Jabbari E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J Controlled Release. 2016;223:126–136. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

