Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis
- PMID: 28710437
- PMCID: PMC5511333
- DOI: 10.1038/s41598-017-05624-2
Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis
Abstract
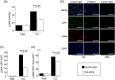
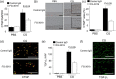
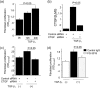
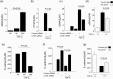
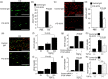
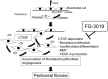
Peritoneal fibrosis (PF) is a serious complication in various clinical settings, but the mechanisms driving it remain to be fully determined. Connective tissue growth factor (CTGF) is known to regulate fibroblast activities. We therefore examined if CTGF inhibition has anti-fibrotic effects in PF. PF was induced by repetitive intraperitoneal injections of chlorhexidine gluconate (CG) in mice with type I pro-collagen promoter-driven green fluorescent protein (GFP) expression to identify fibroblasts. FG-3019, an anti-CTGF monoclonal antibody, was used to inhibit CTGF. CG-induced PF was significantly attenuated in FG-3019-treated mice. CG challenges induced marked accumulations of proliferating fibroblasts and of myofibroblasts, which were both reduced by FG-3019. Levels of peritoneal CTGF expression were increased by CG challenges, and suppressed in FG-3019-treated mice. FG-3019 treatment also reduced the number of CD31+ vessels and VEGF-A-positive cells in fibrotic peritoneum. In vitro studies using NIH 3T3 fibroblasts and peritoneal mesothelial cells (PMCs) showed that CTGF blockade suppressed TGF-β1-induced fibroblast proliferation and myofibroblast differentiation, PMC mesothelial-to-mesenchymal transition, and VEGF-A production. These findings suggest that the inhibition of CTGF by FG-3019 might be a novel treatment for PF through the regulation of fibroblast and myofibroblast accumulation and angiogenesis.
Conflict of interest statement
Dr. Lipson receives income and owns equity in FibroGen Inc.
Figures



Similar articles
-
Deletion of connective tissue growth factor ameliorates peritoneal fibrosis by inhibiting angiogenesis and inflammation.Nephrol Dial Transplant. 2018 Jun 1;33(6):943-953. doi: 10.1093/ndt/gfx317. Nephrol Dial Transplant. 2018. PMID: 29165602
-
Connective tissue growth factor is correlated with peritoneal lymphangiogenesis.Sci Rep. 2019 Aug 21;9(1):12175. doi: 10.1038/s41598-019-48699-9. Sci Rep. 2019. PMID: 31434958 Free PMC article.
-
Therapeutic effect of 1,25(OH)2-VitaminD3 on fibrosis and angiogenesis of peritoneum induced by chlorhexidine.Biomed Pharmacother. 2020 Sep;129:110431. doi: 10.1016/j.biopha.2020.110431. Epub 2020 Jun 22. Biomed Pharmacother. 2020. PMID: 32585450
-
Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition.Int J Mol Sci. 2020 Jun 10;21(11):4158. doi: 10.3390/ijms21114158. Int J Mol Sci. 2020. PMID: 32532126 Free PMC article. Review.
-
Linking myofibroblast generation and microvascular alteration: The role of CD248 from pathogenesis to therapeutic target (Review).Mol Med Rep. 2019 Aug;20(2):1488-1498. doi: 10.3892/mmr.2019.10429. Epub 2019 Jun 26. Mol Med Rep. 2019. PMID: 31257535 Review.
Cited by
-
Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer.Signal Transduct Target Ther. 2021 Jun 10;6(1):218. doi: 10.1038/s41392-021-00641-0. Signal Transduct Target Ther. 2021. PMID: 34108441 Free PMC article. Review.
-
Elucidating the Novel Mechanism of Ligustrazine in Preventing Postoperative Peritoneal Adhesion Formation.Oxid Med Cell Longev. 2022 Mar 10;2022:9226022. doi: 10.1155/2022/9226022. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35308169 Free PMC article.
-
Hyaluronidase-2 Regulates RhoA Signaling, Myofibroblast Contractility, and Other Key Profibrotic Myofibroblast Functions.Am J Pathol. 2020 Jun;190(6):1236-1255. doi: 10.1016/j.ajpath.2020.02.012. Epub 2020 Mar 19. Am J Pathol. 2020. PMID: 32201263 Free PMC article.
-
Engineered stem cell mimics to enhance stroke recovery.Biomaterials. 2018 Sep;178:63-72. doi: 10.1016/j.biomaterials.2018.06.010. Epub 2018 Jun 14. Biomaterials. 2018. PMID: 29909038 Free PMC article.
-
The Inhibition of Adipose-Derived Stem Cells on the Invasion of Keloid Fibroblasts.Int J Med Sci. 2022 Oct 3;19(12):1796-1805. doi: 10.7150/ijms.68646. eCollection 2022. Int J Med Sci. 2022. PMID: 36313222 Free PMC article.
References
-
- Bansal S, et al. Incidence of encapsulating peritoneal sclerosis at a single U.S. university center. Adv Perit Dial. 2010;26:75–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

