Arginase activity in pathogenic and non-pathogenic species of Leishmania parasites
- PMID: 28708893
- PMCID: PMC5529023
- DOI: 10.1371/journal.pntd.0005774
Arginase activity in pathogenic and non-pathogenic species of Leishmania parasites
Abstract
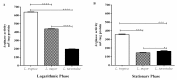
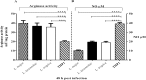
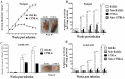
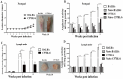
Proliferation of Leishmania (L.) parasites depends on polyamine availability, which can be generated by the L-arginine catabolism and the enzymatic activity of arginase (ARG) of the parasites and of the mammalian hosts. In the present study, we characterized and compared the arginase (arg) genes from pathogenic L. major and L. tropica and from non-pathogenic L. tarentolae. We quantified the level of the ARG activity in promastigotes and macrophages infected with pathogenic L. major and L. tropica and non-pathogenic L. tarentolae amastigotes. The ARG's amino acid sequences of the pathogenic and non-pathogenic Leishmania demonstrated virtually 98.6% and 88% identities with the reference L. major Friedlin ARG. Higher ARG activity was observed in all pathogenic promastigotes as compared to non-pathogenic L. tarentolae. In vitro infection of human macrophage cell line (THP1) with pathogenic and non-pathogenic Leishmania spp. resulted in increased ARG activities in the infected macrophages. The ARG activities present in vivo were assessed in susceptible BALB/c and resistant C57BL/6 mice infected with L. major, L. tropica and L. tarentolae. We demonstrated that during the development of the infection, ARG is induced in both strains of mice infected with pathogenic Leishmania. However, in L. major infected BALB/c mice, the induction of ARG and parasite load increased simultaneously according to the time course of infection, whereas in C57BL/6 mice, the enzyme is upregulated solely during the period of footpad swelling. In L. tropica infected mice, the footpads' swellings were slow to develop and demonstrated minimal cutaneous pathology and ARG activity. In contrast, ARG activity was undetectable in mice inoculated with the non-pathogenic L. tarentolae. Our data suggest that infection by Leishmania parasites can increase ARG activity of the host and provides essential polyamines for parasite salvage and its replication. Moreover, the ARG of Leishmania is vital for parasite proliferation and required for infection in mice. ARG activity can be used as one of the main marker of the disease severity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
L-arginine availability and arginase activity: Characterization of amino acid permease 3 in Leishmania amazonensis.PLoS Negl Trop Dis. 2017 Oct 26;11(10):e0006025. doi: 10.1371/journal.pntd.0006025. eCollection 2017 Oct. PLoS Negl Trop Dis. 2017. PMID: 29073150 Free PMC article.
-
The outcome of arginase activity inhibition in BALB/c mice hosting Leishmania tropica.Parasite Immunol. 2020 Mar;42(3):e12691. doi: 10.1111/pim.12691. Epub 2020 Jan 7. Parasite Immunol. 2020. PMID: 31811772
-
Dual transcriptome analysis reveals differential gene expression modulation influenced by Leishmania arginase and host genetic background.Microb Genom. 2020 Sep;6(9):mgen000427. doi: 10.1099/mgen.0.000427. Epub 2020 Sep 4. Microb Genom. 2020. PMID: 32886592 Free PMC article.
-
L-arginine metabolism and its impact on host immunity against Leishmania infection.Immunol Res. 2008;41(1):15-25. doi: 10.1007/s12026-007-8012-y. Immunol Res. 2008. PMID: 18040886 Free PMC article. Review.
-
Role of trypanosomatid's arginase in polyamine biosynthesis and pathogenesis.Mol Biochem Parasitol. 2012 Feb;181(2):85-93. doi: 10.1016/j.molbiopara.2011.10.007. Epub 2011 Oct 20. Mol Biochem Parasitol. 2012. PMID: 22033378 Review.
Cited by
-
Study of the differentially abundant proteins among Leishmania amazonensis, L. braziliensis, and L. infantum.PLoS One. 2020 Oct 15;15(10):e0240612. doi: 10.1371/journal.pone.0240612. eCollection 2020. PLoS One. 2020. PMID: 33057350 Free PMC article.
-
Leishmania Hijacks Myeloid Cells for Immune Escape.Front Microbiol. 2018 May 7;9:883. doi: 10.3389/fmicb.2018.00883. eCollection 2018. Front Microbiol. 2018. PMID: 29867798 Free PMC article. Review.
-
Leishmania Exosomes/Extracellular Vesicles Containing GP63 Are Essential for Enhance Cutaneous Leishmaniasis Development Upon Co-Inoculation of Leishmania amazonensis and Its Exosomes.Front Cell Infect Microbiol. 2022 Feb 3;11:709258. doi: 10.3389/fcimb.2021.709258. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35186777 Free PMC article.
-
Polyamine Metabolism in Leishmania Parasites: A Promising Therapeutic Target.Med Sci (Basel). 2022 Apr 22;10(2):24. doi: 10.3390/medsci10020024. Med Sci (Basel). 2022. PMID: 35645240 Free PMC article. Review.
-
LmjF.22.0810 from Leishmania major Modulates the Th2-Type Immune Response and Is Involved in Leishmaniasis Outcome.Biomedicines. 2020 Oct 26;8(11):452. doi: 10.3390/biomedicines8110452. Biomedicines. 2020. PMID: 33114674 Free PMC article.
References
-
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671 doi: 10.1371/journal.pone.0035671 - DOI - PMC - PubMed
-
- Abedi-Astaneh F, Hajjaran H, Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Mohebali M, Shirzadi MR, et al. Risk Mapping and Situational Analysis of Cutaneous Leishmaniasis in an Endemic Area of Central Iran: A GIS-Based Survey. PLoS One. 2016;11(8):e0161317 doi: 10.1371/journal.pone.0161317 - DOI - PMC - PubMed
-
- Siriwardana HVYD, Senarath U, Chandrawansa PH, Karunaweera ND. Use of a clinical tool for screening and diagnosis of cutaneous leishmaniasis in Sri Lanka. Pathog Glob Health. 2015;109(4):174–83. doi: 10.1179/2047773215Y.0000000024 - DOI - PMC - PubMed
-
- Karakuş M, Nasereddin A, Onay H, Karaca E, Özkeklikçi A, Jaffe CL, et al. Epidemiological analysis of Leishmania tropica strains and giemsa-stained smears from Syrian and Turkish leishmaniasis patients using multilocus microsatellite typing (MLMT). PLoS Negl Trop Dis. 2017;11(4):e0005538 doi: 10.1371/journal.pntd.0005538 - DOI - PMC - PubMed
-
- Badirzadeh A, Mohebali M, Ghasemian M, Amini H, Zarei Z, Akhoundi B, et al. Cutaneous and post kala-azar dermal leishmaniasis caused by Leishmania infantum in endemic areas of visceral leishmaniasis, northwestern Iran 2002–2011: a case series. Pathog Glob Health. 2013;107(4):194–7. doi: 10.1179/2047773213Y.0000000097 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

