Membrane Association Dictates Ligand Specificity for the Innate Immune Receptor NOD2
- PMID: 28708377
- PMCID: PMC5569645
- DOI: 10.1021/acschembio.7b00469
Membrane Association Dictates Ligand Specificity for the Innate Immune Receptor NOD2
Abstract
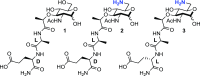
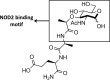
The human gut must regulate its immune response to resident and pathogenic bacteria, numbering in the trillions. The peptidoglycan component of the bacterial cell wall is a dense and rigid structure that consists of polymeric carbohydrates and highly cross-linked peptides which offers protection from the host and surrounding environment. Nucleotide-binding oligomerization domain-containing protein 2 (NOD2), a human membrane-associated innate immune receptor found in the gut epithelium and mutated in an estimated 30% of Crohn's disease patients, binds to peptidoglycan fragments and initiates an immune response. Using a combination of chemical synthesis, advanced analytical assays, and protein biochemistry, we tested the binding of a variety of synthetic peptidoglycan fragments to wild-type (WT)-NOD2. Only when the protein was presented in the native membrane did binding measurements correlate with a NOD2-dependent nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) response, supporting the hypothesis that the native-membrane environment confers ligand specificity to the NOD2 receptor for NF-κB signaling. While N-acetyl-muramyl dipeptide (MDP) has been thought to be the minimal peptidoglycan fragment necessary to activate a NOD2-dependent immune response, we found that fragments with and without the dipeptide moiety are capable of binding and activating a NOD2-dependent NF-κB response, suggesting that the carbohydrate moiety of the peptidoglycan fragments is the minimal functional epitope. This work highlights the necessity of studying NOD2-ligand binding in systems that resemble the receptor's natural environment, as the cellular membrane and/or NOD2 interacting partners appear to play a crucial role in ligand binding and in triggering an innate immune response.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Peptidoglycan Modifications Tune the Stability and Function of the Innate Immune Receptor Nod2.J Am Chem Soc. 2015 Jun 10;137(22):6987-90. doi: 10.1021/jacs.5b01607. Epub 2015 Jun 2. J Am Chem Soc. 2015. PMID: 26035228 Free PMC article.
-
Molecular Recognition of Muramyl Dipeptide Occurs in the Leucine-rich Repeat Domain of Nod2.ACS Infect Dis. 2017 Apr 14;3(4):264-270. doi: 10.1021/acsinfecdis.6b00154. Epub 2016 Oct 31. ACS Infect Dis. 2017. PMID: 27748583 Free PMC article.
-
Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease.J Biol Chem. 2003 Feb 21;278(8):5509-12. doi: 10.1074/jbc.C200673200. Epub 2003 Jan 4. J Biol Chem. 2003. PMID: 12514169
-
Structure-activity relationship in NOD2 agonistic muramyl dipeptides.Eur J Med Chem. 2024 May 5;271:116439. doi: 10.1016/j.ejmech.2024.116439. Epub 2024 Apr 20. Eur J Med Chem. 2024. PMID: 38691886 Review.
-
Nucleotide-binding oligomerization domain containing 2: structure, function, and diseases.Semin Arthritis Rheum. 2013 Aug;43(1):125-30. doi: 10.1016/j.semarthrit.2012.12.005. Epub 2013 Jan 24. Semin Arthritis Rheum. 2013. PMID: 23352252 Review.
Cited by
-
Peptidoglycan Metabolite Photoaffinity Reporters Reveal Direct Binding to Intracellular Pattern Recognition Receptors and Arf GTPases.ACS Chem Biol. 2019 Mar 15;14(3):405-414. doi: 10.1021/acschembio.8b01038. Epub 2019 Feb 20. ACS Chem Biol. 2019. PMID: 30735346 Free PMC article.
-
Synthesis of Bacterial-Derived Peptidoglycan Cross-Linked Fragments.J Org Chem. 2020 Dec 18;85(24):16243-16253. doi: 10.1021/acs.joc.0c01852. Epub 2020 Oct 27. J Org Chem. 2020. PMID: 33108204 Free PMC article.
-
Revisiting peptidoglycan sensing: interactions with host immunity and beyond.Chem Commun (Camb). 2020 Nov 11;56(87):13313-13322. doi: 10.1039/d0cc02605k. Epub 2020 Oct 15. Chem Commun (Camb). 2020. PMID: 33057506 Free PMC article. Review.
-
The immunogenicity and protective immunity of multi-epitopes DNA prime-protein boost vaccines encoding Amastin-Kmp-11, Kmp11-Gp63 and Amastin-Gp63 against visceral leishmaniasis.PLoS One. 2020 Mar 16;15(3):e0230381. doi: 10.1371/journal.pone.0230381. eCollection 2020. PLoS One. 2020. PMID: 32176727 Free PMC article.
-
Crohn's Disease Variants of Nod2 Are Stabilized by the Critical Contact Region of Hsp70.Biochemistry. 2017 Aug 29;56(34):4445-4448. doi: 10.1021/acs.biochem.7b00470. Epub 2017 Aug 15. Biochemistry. 2017. PMID: 28792733 Free PMC article.
References
-
- Lipinski S.; Grabe N.; Jacobs G.; Billmann-Born S.; Till A.; Hasler R.; Aden K.; Paulsen M.; Arlt A.; Kraemer L.; Hagemann N.; Erdmann K. S.; Schreiber S.; Rosenstiel P. (2012) RNAi screening identifies mediators of NOD2 signaling: Implications for spatial specificity of MDP recognition. Proc. Natl. Acad. Sci. U. S. A. 109, 21426–21431. 10.1073/pnas.1209673109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

