Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations
- PMID: 28706010
- PMCID: PMC5599204
- DOI: 10.1634/theoncologist.2017-0142
Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations
Abstract
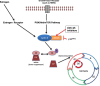
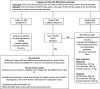
Aberrations of the cell cycle are pervasive in cancer, and selective cell cycle inhibition of cancer cells is a target of choice for a number of novel cancer therapeutics. Cyclin-dependent kinases (CDKs) are key regulatory enzymes that control cell cycle transitions and the commitment to cell division. Palbociclib and ribociclib are both orally active, highly selective reversible inhibitors of CDK4 and CDK6 that are approved by the U.S. Food and Drug Administration (FDA) for hormone receptor-positive metastatic breast cancer in combination with specific endocrine therapies. A third oral CDK4/6 inhibitor, abemaciclib, received Breakthrough Therapy designation status from the FDA and is also being developed in breast cancer. The most common adverse events associated with palbociclib and ribociclib are hematologic, particularly neutropenia. However, the neutropenia associated with CDK4/6 inhibitors is distinct from chemotherapy-induced neutropenia in that it is rapidly reversible, reflecting a cytostatic effect on neutrophil precursors in the bone marrow. Most hematologic abnormalities seen with CDK4/6 inhibitors are not complicated and are adequately managed with standard supportive care and dose adjustments when indicated. Cytopenias are less prevalent with abemaciclib, although fatigue and gastrointestinal toxicity is more common with this agent. This review focuses on the clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer, with a focus on palbociclib and ribociclib, and summarizes practical management strategies for an oncologist.
Implications for practice: The emergence of modern cyclin-dependent kinase (CDK) inhibitors has changed the treatment paradigm for metastatic hormone receptor (HR)-positive breast cancer. Palbociclib, ribociclib, and abemaciclib are highly selective reversible inhibitors of CDK4 and CDK6. Palbociclib is U.S. Food and Drug Administration (FDA)-approved in the first- and second-line settings in combination with endocrine therapy for HR-positive metastatic breast cancer. Ribociclib is FDA-approved in the first-line setting. Abemaciclib has received FDA Breakthrough Therapy designation status. This review focuses on the clinical management of potential toxicities and drug interactions seen with the use of CDK4/6 inhibitors in breast cancer.
摘要
癌症中细胞周期发生改变的情况非常常见, 许多新癌症疗法的目标是选择性抑制癌细胞的细胞周期。细胞周期蛋白依赖性激酶(CDK)是控制细胞周期变化和限定细胞分裂的关键调节酶。Palbociclib和ribociclib均为口服活性的CDK4与CDK6高选择性可逆抑制剂, 已获美国食品药品监督管理局批准用于联合特定内分泌疗法治疗激素受体阳性转移性乳腺癌。第三种口服CDK4/6抑制剂是abemaciclib, 其已经被FDA授予”突破性疗法认定”资格, 目前正被开发用于治疗乳腺癌。与palbociclib和ribociclib相关的最常见不良事件是血液学事件, 尤其是中性粒细胞减少症。然而, 与CDK4/6抑制剂相关的中性粒细胞减少症与化疗诱导的中性粒细胞减少症的不同之处在于前者快速可逆, 反映了其对骨髓中中性粒细胞前体的细胞生长抑制作用。与CDK4/6抑制剂相关的大多数血液学异常并不复杂, 可以通过标准支持性治疗和调整剂量(如必要)进行充分管理。虽然abemaciclib诱发疲乏和胃肠道毒性的情况更为常见, 但导致血细胞减少的情况并不常见。本综述主要论述使用CDK4/6抑制剂(主要为palbociclib和ribociclib)治疗乳腺癌的潜在毒性的临床管理和药物相互作用, 并总结肿瘤医师的实际管理策略。
Keywords: Breast cancer; Cyclin‐dependent kinases; Drug toxicity.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women.Am J Health Syst Pharm. 2019 Aug 1;76(16):1183-1202. doi: 10.1093/ajhp/zxz121. Am J Health Syst Pharm. 2019. PMID: 31369120 Review.
-
Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment.J Oncol Pharm Pract. 2019 Jan;25(1):110-129. doi: 10.1177/1078155218770904. Epub 2018 May 4. J Oncol Pharm Pract. 2019. PMID: 29726787 Review.
-
Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib.Breast Cancer Res Treat. 2017 Nov;166(1):41-54. doi: 10.1007/s10549-017-4385-3. Epub 2017 Jul 24. Breast Cancer Res Treat. 2017. PMID: 28741274 Review.
-
CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib.BioDrugs. 2019 Apr;33(2):125-135. doi: 10.1007/s40259-019-00337-6. BioDrugs. 2019. PMID: 30847853 Review.
-
Biological specificity of CDK4/6 inhibitors: dose response relationship, in vivo signaling, and composite response signature.Oncotarget. 2017 Jul 4;8(27):43678-43691. doi: 10.18632/oncotarget.18435. Oncotarget. 2017. PMID: 28620137 Free PMC article.
Cited by
-
Acute Lymphoblastic Leukemia in a Patient With Advanced Breast Cancer Treated With Cyclin-Dependent Kinase 4/6 Inhibitors and Endocrine Therapy.Cureus. 2024 Sep 16;16(9):e69548. doi: 10.7759/cureus.69548. eCollection 2024 Sep. Cureus. 2024. PMID: 39286469 Free PMC article.
-
Dose reduction and discontinuation due to the combination of CDK4/6 inhibitors and endocrine drugs: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2024 Dec;80(12):1893-1902. doi: 10.1007/s00228-024-03757-8. Epub 2024 Sep 13. Eur J Clin Pharmacol. 2024. PMID: 39271490
-
First‑line endocrine therapy for hormone receptor positive and HER‑2 negative metastatic breast cancer: A Bayesian network meta‑analysis.Oncol Lett. 2024 Aug 28;28(5):513. doi: 10.3892/ol.2024.14646. eCollection 2024 Nov. Oncol Lett. 2024. PMID: 39247493 Free PMC article.
-
Risk of Cardiovascular Events with Cyclin-Dependent Kinases 4 and 6 (CDK 4/6) Inhibitors among Patients with Advanced Breast Cancer: A Systematic Review and Network Meta-Analysis.Rev Cardiovasc Med. 2023 Nov 9;24(11):309. doi: 10.31083/j.rcm2411309. eCollection 2023 Nov. Rev Cardiovasc Med. 2023. PMID: 39076428 Free PMC article.
-
Clinical impact of drug-drug interactions on abemaciclib in the real-world experience of AB-ITALY study.NPJ Breast Cancer. 2024 Jul 17;10(1):58. doi: 10.1038/s41523-024-00657-z. NPJ Breast Cancer. 2024. PMID: 39019916 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

