Autophagy induced by bovine viral diarrhea virus infection counteracts apoptosis and innate immune activation
- PMID: 28702931
- PMCID: PMC7086613
- DOI: 10.1007/s00705-017-3482-2
Autophagy induced by bovine viral diarrhea virus infection counteracts apoptosis and innate immune activation
Abstract
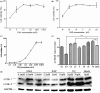
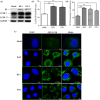
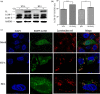
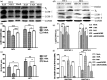
Bovine viral diarrhea virus (BVDV) is an important pathogen of cattle that plays a complex role in disease. There are two biotypes of BVDV: non-cytopathic (NCP) and cytopathic (CP). One strategy that has been used to treat or prevent virus-associated diseases is the modulation of autophagy, which is used by the innate immune system to defend against viral infection; however, at present, the interplay between autophagy and BVDV remains unclear. Madin-Darby bovine kidney cells stably expressing microtubule-associated protein 1 light chain 3B (LC3B) with green fluorescent protein (GFP) (GFP-LC3-MDBK cells) and autophagy-deficient MDBKs (shBCN1-MDBK cells) were constructed. Then MDBK, GFP-LC3-MDBK and shBCN1-MDBK cells were infected with CP or NCP BVDV strains. The LC3-II turnover rate was estimated by western blot, autophagosomes were visualized by confocal microscopy, and ultrastructural analysis was performed using electron microscopy. Autophagy flux was observed using chloroquine as an inhibitor of the autophagic process. The influence of autophagy on BVDV replication and release was investigated using virus titration, and its effect on cell viability was also studied. The effect of BVDV-induced autophagy on the survival of BVDV-infected host cell, cell apoptosis, and interferon (IFN) signalling was studied by flow cytometric analysis and quantitative RT-(q)PCR using shBCN1-MDBK cells. we found that infection with either CP or NCP BVDV strains induced steady-state autophagy in MDBK cells, as evident by the increased number of double- or single-membrane vesicles, the accumulation of GFP- microtubule-associated protein 1 light chain 3 (LC3) dots, and the conversion of LC3-I (cytosolic) to LC3-II (membrane-bound) forms. The complete autophagic process was verified by monitoring the LC3-II turnover ratio, lysosomal delivery, and proteolysis. In addition, we found that CP and NCP BVDV growth was inhibited in MDBK cells treated with high levels of an autophagy inducer or inhibitor, or in autophagy deficient-MDBK cells. Furthermore, our studies also suggested that CP and NCP BVDV infection in autophagy-knockdown MDBK cells increased apoptotic cell death and enhanced the expression of the mRNAs for IFN-α, Mx1, IFN-β, and OAS-1 as compared with control MDBK cells. Our study provides strong evidence that BVDV infection induces autophagy, which facilitates BVDV replication in MDBK cells and impairs the innate immune response. These findings might help to illustrate the pathogenesis of persistent infection caused by BVDV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Non-cytopathic bovine viral diarrhoea virus 2 induces autophagy to enhance its replication.Vet Med Sci. 2023 Jan;9(1):405-416. doi: 10.1002/vms3.1052. Epub 2022 Dec 19. Vet Med Sci. 2023. PMID: 36533845 Free PMC article.
-
Both cytopathic and non-cytopathic bovine viral diarrhea virus (BVDV) induced autophagy at a similar rate.Vet Immunol Immunopathol. 2017 Dec;193-194:1-9. doi: 10.1016/j.vetimm.2017.09.006. Epub 2017 Oct 2. Vet Immunol Immunopathol. 2017. PMID: 29129222
-
Autophagy during early stages contributes to bovine viral diarrhea virus replication in MDBK cells.J Basic Microbiol. 2014 Oct;54(10):1044-52. doi: 10.1002/jobm.201300750. Epub 2013 Dec 17. J Basic Microbiol. 2014. PMID: 24347372
-
Bovine viral diarrhea virus infection induces autophagy in MDBK cells.J Microbiol. 2014 Jul;52(7):619-25. doi: 10.1007/s12275-014-3479-4. Epub 2014 Jun 28. J Microbiol. 2014. PMID: 24972811 Review.
-
Research advances on interferon (IFN) response during BVDV infection.Res Vet Sci. 2022 Dec;149:151-158. doi: 10.1016/j.rvsc.2022.04.011. Epub 2022 Jun 24. Res Vet Sci. 2022. PMID: 35839708 Review.
Cited by
-
Autophagy in farm animals: current knowledge and future challenges.Autophagy. 2021 Aug;17(8):1809-1827. doi: 10.1080/15548627.2020.1798064. Epub 2020 Jul 30. Autophagy. 2021. PMID: 32686564 Free PMC article. Review.
-
Temporal Dynamics of the Ruminant Type I IFN-Induced Antiviral State against Homologous Parainfluenza Virus 3 Challenge In Vitro.Viruses. 2022 May 11;14(5):1025. doi: 10.3390/v14051025. Viruses. 2022. PMID: 35632770 Free PMC article.
-
Non-cytopathic bovine viral diarrhoea virus 2 induces autophagy to enhance its replication.Vet Med Sci. 2023 Jan;9(1):405-416. doi: 10.1002/vms3.1052. Epub 2022 Dec 19. Vet Med Sci. 2023. PMID: 36533845 Free PMC article.
-
Rapamycin recruits SIRT2 for FKBP12 deacetylation during mTOR activity modulation in innate immunity.iScience. 2021 Sep 27;24(11):103177. doi: 10.1016/j.isci.2021.103177. eCollection 2021 Nov 19. iScience. 2021. PMID: 34712915 Free PMC article.
-
Mitochondria-mediated ferroptosis contributes to the inflammatory responses of bovine viral diarrhea virus (BVDV) in vitro.J Virol. 2024 Feb 20;98(2):e0188023. doi: 10.1128/jvi.01880-23. Epub 2024 Jan 16. J Virol. 2024. PMID: 38226812 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

