Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection
- PMID: 28698273
- PMCID: PMC5513709
- DOI: 10.1128/mBio.00576-17
Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection
Abstract
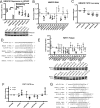
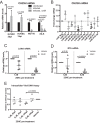
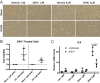
From various screens, we found that Kaposi's sarcoma-associated herpesvirus (KSHV) viral microRNAs (miRNAs) target several enzymes in the mevalonate/cholesterol pathway. 3-Hydroxy-3-methylglutaryl-coenzyme A (CoA) synthase 1 (HMGCS1), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR [a rate-limiting step in the mevalonate pathway]), and farnesyl-diphosphate farnesyltransferase 1 (FDFT1 [a committed step in the cholesterol branch]) are repressed by multiple KSHV miRNAs. Transfection of viral miRNA mimics in primary endothelial cells (human umbilical vein endothelial cells [HUVECs]) is sufficient to reduce intracellular cholesterol levels; however, small interfering RNAs (siRNAs) targeting only HMGCS1 did not reduce cholesterol levels. This suggests that multiple targets are needed to perturb this tightly regulated pathway. We also report here that cholesterol levels were decreased in de novo-infected HUVECs after 7 days. This reduction is at least partially due to viral miRNAs, since the mutant form of KSHV lacking 10 of the 12 miRNA genes had increased cholesterol compared to wild-type infections. We hypothesized that KSHV is downregulating cholesterol to suppress the antiviral response by a modified form of cholesterol, 25-hydroxycholesterol (25HC). We found that the cholesterol 25-hydroxylase (CH25H) gene, which is responsible for generating 25HC, had increased expression in de novo-infected HUVECs but was strongly suppressed in long-term latently infected cell lines. We found that 25HC inhibits KSHV infection when added exogenously prior to de novo infection. In conclusion, we found that multiple KSHV viral miRNAs target enzymes in the mevalonate pathway to modulate cholesterol in infected cells during latency. This repression of cholesterol levels could potentially be beneficial to viral infection by decreasing the levels of 25HC.IMPORTANCE A subset of viruses express unique microRNAs (miRNAs), which act like cellular miRNAs to generally repress host gene expression. A cancer virus, Kaposi's sarcoma-associated herpesvirus (KSHV, or human herpesvirus 8 [HHV-8]), encodes multiple miRNAs that repress gene expression of multiple enzymes that are important for cholesterol synthesis. In cells with these viral miRNAs or with natural infection, cholesterol levels are reduced, indicating these viral miRNAs decrease cholesterol levels. A modified form of cholesterol, 25-hydroxycholesterol, is generated directly from cholesterol. Addition of 25-hydroxycholesterol to primary cells inhibited KSHV infection of cells, suggesting that viral miRNAs may decrease cholesterol levels to decrease the concentration of 25-hydroxycholesterol and to promote infection. These results suggest a new virus-host relationship and indicate a previously unidentified viral strategy to lower cholesterol levels.
Keywords: Kaposi's sarcoma-associated herpesvirus; cholesterol; human herpesviruses; microRNA.
Copyright © 2017 Serquiña et al.
Figures


Similar articles
-
25-Hydroxycholesterol Inhibits Kaposi's Sarcoma Herpesvirus and Epstein-Barr Virus Infections and Activates Inflammatory Cytokine Responses.mBio. 2021 Dec 21;12(6):e0290721. doi: 10.1128/mBio.02907-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781692 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus MicroRNAs Target GADD45B To Protect Infected Cells from Cell Cycle Arrest and Apoptosis.J Virol. 2017 Jan 18;91(3):e02045-16. doi: 10.1128/JVI.02045-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27852859 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus microRNAs repress breakpoint cluster region protein expression, enhance Rac1 activity, and increase in vitro angiogenesis.J Virol. 2015 Apr;89(8):4249-61. doi: 10.1128/JVI.03687-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631082 Free PMC article.
-
miRNAs and their roles in KSHV pathogenesis.Virus Res. 2019 Jun;266:15-24. doi: 10.1016/j.virusres.2019.03.024. Epub 2019 Apr 2. Virus Res. 2019. PMID: 30951791 Review.
-
KSHV microRNAs: Tricks of the Devil.Trends Microbiol. 2017 Aug;25(8):648-661. doi: 10.1016/j.tim.2017.02.002. Epub 2017 Mar 2. Trends Microbiol. 2017. PMID: 28259385 Free PMC article. Review.
Cited by
-
Viral non-coding RNAs: Stealth strategies in the tug-of-war between humans and herpesviruses.Semin Cell Dev Biol. 2021 Mar;111:135-147. doi: 10.1016/j.semcdb.2020.06.015. Epub 2020 Jul 3. Semin Cell Dev Biol. 2021. PMID: 32631785 Free PMC article. Review.
-
Curbing Lipids: Impacts ON Cancer and Viral Infection.Int J Mol Sci. 2019 Feb 2;20(3):644. doi: 10.3390/ijms20030644. Int J Mol Sci. 2019. PMID: 30717356 Free PMC article. Review.
-
The Oxysterol 25-Hydroxycholesterol Inhibits Replication of Murine Norovirus.Viruses. 2019 Jan 24;11(2):97. doi: 10.3390/v11020097. Viruses. 2019. PMID: 30682775 Free PMC article.
-
Studies in the antiviral molecular mechanisms of 25-hydroxycholesterol: Disturbing cholesterol homeostasis and post-translational modification of proteins.Eur J Pharmacol. 2022 Jul 5;926:175033. doi: 10.1016/j.ejphar.2022.175033. Epub 2022 May 19. Eur J Pharmacol. 2022. PMID: 35598845 Free PMC article. Review.
-
Statins significantly repress rotavirus replication through downregulation of cholesterol synthesis.Gut Microbes. 2021 Jan-Dec;13(1):1955643. doi: 10.1080/19490976.2021.1955643. Gut Microbes. 2021. Retraction in: Gut Microbes. 2022 Jan-Dec;14(1):2151146. doi: 10.1080/19490976.2022.2151146 PMID: 34369301 Free PMC article. Retracted.
References
-
- Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, Watterson S, Griffiths SJ, Spann NJ, Meljon A, Talbot S, Krishnan K, Covey DF, Wenk MR, Craigon M, Ruzsics Z, Haas J, Angulo A, Griffiths WJ, Glass CK, Wang Y, Ghazal P. 2013. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38:106–118. doi:10.1016/j.immuni.2012.11.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
