An Insight into Glyco-Microheterogeneity of Plasma von Willebrand Factor by Mass Spectrometry
- PMID: 28696719
- PMCID: PMC6309539
- DOI: 10.1021/acs.jproteome.7b00359
An Insight into Glyco-Microheterogeneity of Plasma von Willebrand Factor by Mass Spectrometry
Abstract
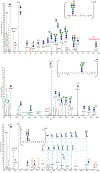
Human plasma von Willebrand Factor (VWF) plays essential roles in primary hemostasis in cooperation with other coagulations factors. There is ample indication that glycosylation affects many biological phases during the protein life cycle. However, comprehensive characterization of all probable N-glycosites simultaneous with O-glycosites is still not fully revealed. Thus, the intention of this exploration was to estimate the occupancy of all canonical N-glycosites besides simultaneous characterization of N- and O-glycoforms. An RP-LC-MS/MS system functionalized with CID and HCD tandem mass was utilized to analyze VWF. N-Glycosite occupancy varied along the protein backbone chain. Out of 257 HCD spectra, 181 characterized glycoforms were specified as either N- or O-glycosites. Sequential cleavage of glycosidic bonds along with Human Database mass matching have confirmed the glycoform structures. A total of 173 glycoforms represented most commonly biantennary and infrequently tri- and tetra-antennary N-glycans beside high mannose, hybrid, ABH antigen-terminated, and sulfated N-glycans. Many glycoforms were common across all N-sites. Noteworthy, previously unreported N-glycosites within domain D'(TIL'-E') showed glycosylation. Moreover, sialylated core 1 and core 2 O-glycans were detected on 2298T. Given subtle characterization of site-specific glycoforms, we can attain a profound understanding of the biological roles of VWF as well as facilitate the production of VWF-based therapeutics.
Keywords: CID; HCD; N-glycan; O-glycan; VWF; glycosylation; mass spectrometry; microheterogeneity; occupancy; plasma von Willebrand Factor.
Figures

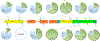

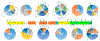




Similar articles
-
GPQuest: A Spectral Library Matching Algorithm for Site-Specific Assignment of Tandem Mass Spectra to Intact N-glycopeptides.Anal Chem. 2015;87(10):5181-8. doi: 10.1021/acs.analchem.5b00024. Epub 2015 May 6. Anal Chem. 2015. PMID: 25945896 Free PMC article.
-
Site-specific analysis of von Willebrand factor O-glycosylation.J Thromb Haemost. 2016 Apr;14(4):733-46. doi: 10.1111/jth.13260. Epub 2016 Feb 17. J Thromb Haemost. 2016. PMID: 26784534
-
The plasma von Willebrand factor O-glycome comprises a surprising variety of structures including ABH antigens and disialosyl motifs.J Thromb Haemost. 2010 Jan;8(1):137-45. doi: 10.1111/j.1538-7836.2009.03665.x. Epub 2009 Oct 24. J Thromb Haemost. 2010. PMID: 19874459
-
von Willebrand factor sialylation-A critical regulator of biological function.J Thromb Haemost. 2019 Jul;17(7):1018-1029. doi: 10.1111/jth.14471. Epub 2019 May 30. J Thromb Haemost. 2019. PMID: 31055873 Review.
-
The Biological Significance of von Willebrand Factor O-Linked Glycosylation.Semin Thromb Hemost. 2021 Oct;47(7):855-861. doi: 10.1055/s-0041-1726373. Epub 2021 Jun 15. Semin Thromb Hemost. 2021. PMID: 34130346 Review.
Cited by
-
Combination of CLEC4M rs868875 G-Carriership and ABO O Genotypes May Predict Faster Decay of FVIII Infused in Hemophilia A Patients.J Clin Med. 2022 Jan 29;11(3):733. doi: 10.3390/jcm11030733. J Clin Med. 2022. PMID: 35160186 Free PMC article.
-
Site-Specific N-Glycosylation on the AAV8 Capsid Protein.Viruses. 2018 Nov 17;10(11):644. doi: 10.3390/v10110644. Viruses. 2018. PMID: 30453606 Free PMC article.
-
Comprehensive N- and O-glycosylation mapping of human coagulation factor V.J Thromb Haemost. 2020 Aug;18(8):1884-1892. doi: 10.1111/jth.14861. Epub 2020 Jun 14. J Thromb Haemost. 2020. PMID: 32310329 Free PMC article.
-
Sialylation on O-linked glycans protects von Willebrand factor from macrophage galactose lectin-mediated clearance.Haematologica. 2022 Mar 1;107(3):668-679. doi: 10.3324/haematol.2020.274720. Haematologica. 2022. PMID: 33763999 Free PMC article.
-
Quantification and site-specific analysis of co-occupied N- and O-glycopeptides.bioRxiv [Preprint]. 2024 Jul 7:2024.07.06.602348. doi: 10.1101/2024.07.06.602348. bioRxiv. 2024. Update in: J Proteome Res. 2024 Dec 6;23(12):5449-5461. doi: 10.1021/acs.jproteome.4c00574. PMID: 39005468 Free PMC article. Updated. Preprint.
References
-
- Varki A; Esko JD; Colley KJ Cellular Organization of Glycosylation In Essentials of Glycobiology, 2nd ed.; Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Eds.; Cold Spring Harbor: New York, NY, 2009. - PubMed
-
- Baruch D; Bahnak B; Girma JP; Meyer D von Willebrand factor and platelet function. Bailliere’s clinical haematology 1989, 2 (3), 627–72. - PubMed
-
- Wise RJ; Dorner AJ; Krane M; Pittman DD; Kaufman RJ The role of von Willebrand factor multimers and propeptide cleavage in binding and stabilization of factor VIII. J. Biol. Chem 1991, 266 (32), 21948–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

