Combination Therapy with Isavuconazole and Micafungin for Treatment of Experimental Invasive Pulmonary Aspergillosis
- PMID: 28696236
- PMCID: PMC5571280
- DOI: 10.1128/AAC.00305-17
Combination Therapy with Isavuconazole and Micafungin for Treatment of Experimental Invasive Pulmonary Aspergillosis
Abstract
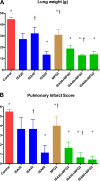
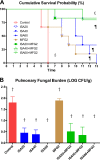
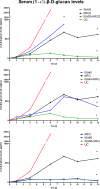
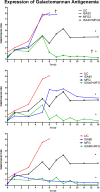
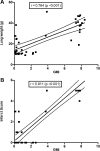
Invasive pulmonary aspergillosis (IPA) is an important cause of morbidity and mortality in immunocompromised patients. We hypothesized that simultaneous inhibition of biosynthesis of ergosterol in the fungal cell membrane and (1→3)-β-d-glucan in the cell wall, respectively, by the antifungal triazole isavuconazole (ISA) and the echinocandin micafungin (MFG) may result in improved outcomes in experimental IPA in persistently neutropenic rabbits. Treatments included ISA at 20 mg/kg of body weight/day (ISA20), 40 mg/kg/day (ISA40), and 60 mg/kg/day (ISA60); MFG at 2 mg/kg/day (MFG2); combinations of ISA20 and MFG2, ISA40 and MFG2, and ISA60 and MFG2; and no treatment (untreated controls [UC]). The galactomannan index (GMI) and (1→3)-β-d-glucan levels in serum were measured. The residual fungal burden (number of CFU per gram) was significantly reduced in ISA20-, ISA40-, ISA60-, ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits compared with that in MFG2-treated or UC rabbits (P < 0.01). Measures of organism-mediated pulmonary injury, lung weights, and pulmonary infarct score were lower in ISA40-MFG2-treated rabbits than in rabbits treated with ISA40 or MFG2 alone (P < 0.01). Survival was prolonged in ISA40-MFG2-treated rabbits in comparison to those treated with ISA40 or MFG2 alone (P < 0.01). These outcome variables correlated directly with significant declines in GMI and serum (1→3)-β-d-glucan levels during therapy. The GMI correlated with measures of organism-mediated pulmonary injury, lung weights (r = 0.764; P < 0.001), and pulmonary infarct score (r = 0.911; P < 0.001). In summary, rabbits receiving combination therapy with isavuconazole and micafungin demonstrated a significant dose-dependent reduction in the residual fungal burden, decreased pulmonary injury, prolonged survival, a lower GMI, and lower serum (1→3)-β-d-glucan levels in comparison to rabbits receiving isavuconazole or micafungin as a single agent.
Keywords: (1→3)-β-d-glucan; Aspergillus fumigatus; aspergillosis; combination therapy; galactomannan; isavuconazole; micafungin; neutropenia; pharmacokinetics; rabbit.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?J Fungi (Basel). 2020 Sep 30;6(4):198. doi: 10.3390/jof6040198. J Fungi (Basel). 2020. PMID: 33007839 Free PMC article. Review.
-
Pharmacokinetics and Concentration-Dependent Efficacy of Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis.Antimicrob Agents Chemother. 2016 Apr 22;60(5):2718-26. doi: 10.1128/AAC.02665-15. Print 2016 May. Antimicrob Agents Chemother. 2016. PMID: 26883703 Free PMC article.
-
Combination Therapy with Ibrexafungerp (Formerly SCY-078), a First-in-Class Triterpenoid Inhibitor of (1→3)-β-d-Glucan Synthesis, and Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis.Antimicrob Agents Chemother. 2020 May 21;64(6):e02429-19. doi: 10.1128/AAC.02429-19. Print 2020 May 21. Antimicrob Agents Chemother. 2020. PMID: 32179521 Free PMC article.
-
Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia.Antimicrob Agents Chemother. 2002 Jan;46(1):12-23. doi: 10.1128/AAC.46.1.12-23.2002. Antimicrob Agents Chemother. 2002. PMID: 11751105 Free PMC article.
-
[Micafungin: experimental therapy of fungal infections in animal models].Rev Iberoam Micol. 2009 Mar 31;26(1):42-8. doi: 10.1016/S1130-1406(09)70007-5. Epub 2009 May 7. Rev Iberoam Micol. 2009. PMID: 19463276 Review. Spanish.
Cited by
-
Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?J Fungi (Basel). 2020 Sep 30;6(4):198. doi: 10.3390/jof6040198. J Fungi (Basel). 2020. PMID: 33007839 Free PMC article. Review.
-
Isavuconazole for Treating Invasive Mould Disease in Solid Organ Transplant Recipients.Transpl Int. 2023 Dec 15;36:11845. doi: 10.3389/ti.2023.11845. eCollection 2023. Transpl Int. 2023. PMID: 38161768 Free PMC article. Review.
-
Diagnostic Aspects of Veterinary and Human Aspergillosis.Front Microbiol. 2018 Jun 21;9:1303. doi: 10.3389/fmicb.2018.01303. eCollection 2018. Front Microbiol. 2018. PMID: 29977229 Free PMC article.
-
Tackling the emerging threat of antifungal resistance to human health.Nat Rev Microbiol. 2022 Sep;20(9):557-571. doi: 10.1038/s41579-022-00720-1. Epub 2022 Mar 29. Nat Rev Microbiol. 2022. PMID: 35352028 Free PMC article. Review.
-
Challenges in the Treatment of Invasive Aspergillosis in Immunocompromised Children.Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0215621. doi: 10.1128/aac.02156-21. Epub 2022 Jun 29. Antimicrob Agents Chemother. 2022. PMID: 35766509 Free PMC article. Review.
References
-
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi:10.1086/651263. - DOI - PubMed
-
- Rubio PM, Sevilla J, Gonzalez-Vicent M, Lassaletta A, Cuenca-Estrella M, Diaz MA, Riesco S, Madero L. 2009. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: a retrospective single centre study. J Pediatr Hematol Oncol 31:642–646. doi:10.1097/MPH.0b013e3181acd956. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

