A Tiny RNA that Packs a Big Punch: The Critical Role of a Viral miR-155 Ortholog in Lymphomagenesis in Marek's Disease
- PMID: 28694799
- PMCID: PMC5483433
- DOI: 10.3389/fmicb.2017.01169
A Tiny RNA that Packs a Big Punch: The Critical Role of a Viral miR-155 Ortholog in Lymphomagenesis in Marek's Disease
Abstract
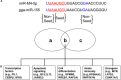
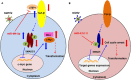
MicroRNAs (miRNAs) are small non-coding RNAs that have been identified in animals, plants, and viruses. These small RNAs play important roles in post-transcriptional regulation of various cellular processes, including development, differentiation, and all aspects of cancer biology. Rapid-onset T-cell lymphoma of chickens, namely Marek's disease (MD), induced by Gallid alphaherpesvirus 2 (GaHV2), could provide an ideal natural animal model for herpesvirus-related cancer research. GaHV2 encodes 26 mature miRNAs derived from 14 precursors assembled in three distinct gene clusters in the viral genome. One of the most highly expressed GaHV2 miRNAs, miR-M4-5p, shows high sequence similarity to the cellular miR-155 and the miR-K12-11 encoded by Kaposi's sarcoma-associated herpesvirus, particularly in the miRNA "seed region." As with miR-K12-11, miR-M4-5p shares a common set of host and viral target genes with miR-155, suggesting that they may target the same regulatory cellular networks; however, differences in regulatory function between miR-155 and miR-M4-5p may distinguish non-viral and viral mediated tumorigenesis. In this review, we focus on the functions of miR-M4-5p as the viral ortholog of miR-155 to explore how the virus mimics a host pathway to benefit the viral life cycle and trigger virus-induced tumorigenesis.
Keywords: GaHV2; Marek’s disease virus; herpesvirus; miR-155; miR-M4-5p; pathogenesis; tumorigenesis.
Figures
Similar articles
-
Marek's Disease Virus-Encoded MicroRNA 155 Ortholog Critical for the Induction of Lymphomas Is Not Essential for the Proliferation of Transformed Cell Lines.J Virol. 2019 Aug 13;93(17):e00713-19. doi: 10.1128/JVI.00713-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189706 Free PMC article.
-
Putative roles as oncogene or tumour suppressor of the Mid-clustered microRNAs in Gallid alphaherpesvirus 2 (GaHV2) induced Marek's disease lymphomagenesis.J Gen Virol. 2017 May;98(5):1097-1112. doi: 10.1099/jgv.0.000786. Epub 2017 May 11. J Gen Virol. 2017. PMID: 28510513 Free PMC article.
-
Virus-encoded miR-155 ortholog in Marek's disease virus promotes cell proliferation via suppressing apoptosis by targeting tumor suppressor WWOX.Vet Microbiol. 2021 Jan;252:108919. doi: 10.1016/j.vetmic.2020.108919. Epub 2020 Nov 7. Vet Microbiol. 2021. PMID: 33191002
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Current state of Marek's disease virus microRNA research.Avian Dis. 2013 Jun;57(2 Suppl):332-9. doi: 10.1637/10355-090812-Review.1. Avian Dis. 2013. PMID: 23901744 Review.
Cited by
-
The Transcriptional Landscape of Marek's Disease Virus in Primary Chicken B Cells Reveals Novel Splice Variants and Genes.Viruses. 2019 Mar 16;11(3):264. doi: 10.3390/v11030264. Viruses. 2019. PMID: 30884829 Free PMC article.
-
Multifunctional miR-155 Pathway in Avian Oncogenic Virus-Induced Neoplastic Diseases.Noncoding RNA. 2019 Mar 13;5(1):24. doi: 10.3390/ncrna5010024. Noncoding RNA. 2019. PMID: 30871221 Free PMC article. Review.
-
Latest Insights into Marek's Disease Virus Pathogenesis and Tumorigenesis.Cancers (Basel). 2020 Mar 10;12(3):647. doi: 10.3390/cancers12030647. Cancers (Basel). 2020. PMID: 32164311 Free PMC article. Review.
-
Effect of gga-miR-155 on cell proliferation, apoptosis and invasion of Marek's disease virus (MDV) transformed cell line MSB1 by targeting RORA.BMC Vet Res. 2020 Jan 28;16(1):23. doi: 10.1186/s12917-020-2239-4. BMC Vet Res. 2020. PMID: 31992293 Free PMC article.
-
Marek's Disease Virus-Encoded MicroRNA 155 Ortholog Critical for the Induction of Lymphomas Is Not Essential for the Proliferation of Transformed Cell Lines.J Virol. 2019 Aug 13;93(17):e00713-19. doi: 10.1128/JVI.00713-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189706 Free PMC article.
References
-
- Amor S., Strassheim S., Dambrine G., Remy S., Rasschaert D., Laurent S. (2011). ICP27 protein of Marek’s disease virus interacts with SR proteins and inhibits the splicing of cellular telomerase chTERT and viral vIL8 transcripts. J. Gen. Virol. 92(Pt 6) 1273–1278. 10.1099/vir.0.028969-0 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

