Characterization of γδ T cells in patients with non-small cell lung cancer
- PMID: 28693285
- PMCID: PMC5494795
- DOI: 10.3892/ol.2017.6191
Characterization of γδ T cells in patients with non-small cell lung cancer
Abstract
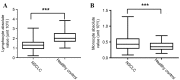
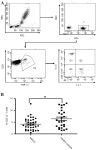
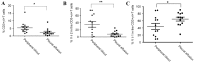
Systemic immune defects that are associated with disease progression exist in a variety of malignancies. γδ T cells are innate-like lymphocytes that do not require self-major histocompatibility complex-restricted priming. Ex vivo-expanded circulating γδ T cells exhibit promising antitumor activity and are a potential candidate for the treatment of various malignancies, including non-small cell lung cancer (NSCLC). In the present study, flow cytometry was used as a method to study the phenotypes and characteristics of γδ T cells. A lower frequency of circulating γδ T cells was observed in NSCLC patients than in healthy controls. In advanced NSCLC patients, γδ T cells were also detected in the pleural effusion, but the frequency of γδ T cells here was significantly lower than in the peripheral blood. Vδ1+and Vδ1-Vδ2- T cells represented the most enriched subsets in the pleural effusion. Moreover, the present study demonstrated that Vδ1+ T cells are a type of γδ T cells characterized by a cluster of differentiation (CD)3dim T-cell receptor (TCR)γδbright phenotype, whereas Vδ2+ T cells represent a CD3brightTCRγδdim phenotype, according to the fluorescence intensity of CD3 and γδTCR using flow cytometry. Finally, the present study reported a decrease in the expression of CD27 and CD28 molecules on the surface of circulating γδ T cells in NSCLC. The present data suggest the existence of a dysregulated repertoire of γδ T cells in NSCLC, which exhibit impaired activation and a reformed cytokine-releasing profile. Although the ex vivo expansion of γδ T cells may be a prospective therapeutic strategy in NSCLC patients, it remains necessary to clarify which subsets (Vδ1 or Vδ2) should be expanded and the sources from which γδ T cells should be generated.
Keywords: non-small cell lung cancer; peripheral blood; pleural effusion; γδ T cells.
Figures


Similar articles
-
Aberrant phenotypes of circulating γδ-T cells may be involved in the onset of systemic lupus erythematosus.Lupus. 2024 May;33(6):587-597. doi: 10.1177/09612033241240864. Epub 2024 Mar 20. Lupus. 2024. PMID: 38506324
-
Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood.Cancer Biol Ther. 2009 Aug;8(16):1540-9. doi: 10.4161/cbt.8.16.8950. Epub 2009 Aug 8. Cancer Biol Ther. 2009. PMID: 19471115
-
[Characteristics of γδ T cell subsets induced from peripheral blood mononuclear cells of HIV/AIDS patients in vitro].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012 Mar;28(3):285-7. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012. PMID: 22394638 Chinese.
-
Heterogeneity of Human γδ T Cells and Their Role in Cancer Immunity.Immune Netw. 2020 Feb 14;20(1):e5. doi: 10.4110/in.2020.20.e5. eCollection 2020 Feb. Immune Netw. 2020. PMID: 32158593 Free PMC article. Review.
-
Role of gammadelta T lymphocytes in tumor defense.Front Biosci. 2004 Sep 1;9:2588-604. doi: 10.2741/1419. Front Biosci. 2004. PMID: 15358583 Review.
Cited by
-
Landscape of unconventional γδ T cell subsets in cancer.Mol Biol Rep. 2024 Jan 30;51(1):238. doi: 10.1007/s11033-024-09267-1. Mol Biol Rep. 2024. PMID: 38289417 Review.
-
Long-term prognostic significance of interleukin-17-producing T cells in patients with non-small cell lung cancer.Cancer Sci. 2019 Jul;110(7):2100-2109. doi: 10.1111/cas.14068. Epub 2019 Jun 28. Cancer Sci. 2019. PMID: 31100180 Free PMC article.
-
Immunization against ROS1 by DNA Electroporation Impairs K-Ras-Driven Lung Adenocarcinomas .Vaccines (Basel). 2020 Apr 6;8(2):166. doi: 10.3390/vaccines8020166. Vaccines (Basel). 2020. PMID: 32268572 Free PMC article.
-
Elevated CD3low double negative T lymphocyte is associated with pneumonia and its severity in pediatric patients.PeerJ. 2018 Dec 18;6:e6114. doi: 10.7717/peerj.6114. eCollection 2018. PeerJ. 2018. PMID: 30588404 Free PMC article.
-
γδT Cells in Lung Cancer Malignant Pleural Effusion: Friend? Foe?Am J Respir Cell Mol Biol. 2019 Aug;61(2):130-131. doi: 10.1165/rcmb.2019-0080ED. Am J Respir Cell Mol Biol. 2019. PMID: 30958972 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
