Interstitial Pneumonitis Secondary to Trastuzumab: A Case Report and Literature Review
- PMID: 28690527
- PMCID: PMC5498939
- DOI: 10.1159/000477340
Interstitial Pneumonitis Secondary to Trastuzumab: A Case Report and Literature Review
Abstract
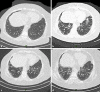
Interstitial lung disease is a rare complication of trastuzumab-based breast cancer treatment with few case reports published. Herein, we report the case of a 67-year-old female with early-stage HER2-postitive breast cancer who developed interstitial pneumonitis during cycle 5 of treatment with trastuzumab combined with carboplatin and docetaxel. After supportive care and treatment with prednisone, the patient showed rapid improvement of respiratory symptoms. Retreatment with trastuzumab as a single agent led to worsening of symptoms and required a second course of treatment with prednisone combined with cyclophosphamide, which was followed by improvement of symptoms. In conclusion, interstitial pneumonitis is a rare but life-threatening adverse event from trastuzumab breast cancer treatment.
Keywords: Breast cancer; Pneumonitis; Toxicity; Trastuzumab.
Figures
Similar articles
-
Trastuzumab-Induced Interstitial Pneumonitis.Cureus. 2023 Jul 19;15(7):e42116. doi: 10.7759/cureus.42116. eCollection 2023 Jul. Cureus. 2023. PMID: 37602136 Free PMC article.
-
Interstitial lung disease associated with trastuzumab monotherapy: A report of 3 cases.Mol Clin Oncol. 2017 Feb;6(2):229-232. doi: 10.3892/mco.2016.1113. Epub 2016 Dec 19. Mol Clin Oncol. 2017. PMID: 28357100 Free PMC article.
-
Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial.
-
HER2-positive breast cancer: update on Breast Cancer International Research Group trials.Clin Breast Cancer. 2002 Oct;3 Suppl 2:S75-9. doi: 10.3816/cbc.2002.s.016. Clin Breast Cancer. 2002. PMID: 12435291 Review.
-
A commentary on interstitial pneumonitis induced by docetaxel: clinical cases and systematic review of the literature.Tumori. 2015 Jun 25;101(3):e92-5. doi: 10.5301/tj.5000275. Tumori. 2015. PMID: 25908033 Review.
Cited by
-
Trastuzumab-Induced Interstitial Pneumonitis.Cureus. 2023 Jul 19;15(7):e42116. doi: 10.7759/cureus.42116. eCollection 2023 Jul. Cureus. 2023. PMID: 37602136 Free PMC article.
-
The first reported case of trastuzumab induced interstitial lung disease associated with anti-neutrophil cytoplasmic antibody vasculitis - A case report and a prospective cohort study on the usefulness of neutrophil derived biomarkers in monitoring vasculitis disease activity during follow-up.Breast. 2022 Feb;61:35-42. doi: 10.1016/j.breast.2021.11.016. Epub 2021 Nov 29. Breast. 2022. PMID: 34894465 Free PMC article.
-
Drug-Related Pneumonitis in Cancer Treatment during the COVID-19 Era.Cancers (Basel). 2021 Mar 2;13(5):1052. doi: 10.3390/cancers13051052. Cancers (Basel). 2021. PMID: 33801385 Free PMC article. Review.
-
Ado-Trastuzumab Emtansine-Induced Pulmonary Toxicity: A Single-Institution Retrospective Review.Case Rep Oncol. 2018 Aug 9;11(2):527-533. doi: 10.1159/000491574. eCollection 2018 May-Aug. Case Rep Oncol. 2018. PMID: 30186135 Free PMC article.
-
Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys.Cancer Sci. 2020 Dec;111(12):4636-4645. doi: 10.1111/cas.14686. Epub 2020 Nov 2. Cancer Sci. 2020. PMID: 33051938 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest. 2006;129:1031–1038. - PubMed
-
- Costa RB, Kurra G, Greenberg L, Geyer CE. Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol. 2010;21:2153–2160. - PubMed
-
- Snyder LS, Hertz MI. Cytotoxic drug-induced lung injury. Semin Respir Infect. 1988;3:217–228. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

