Mitochondrial Uncoupler Prodrug of 2,4-Dinitrophenol, MP201, Prevents Neuronal Damage and Preserves Vision in Experimental Optic Neuritis
- PMID: 28680531
- PMCID: PMC5478871
- DOI: 10.1155/2017/7180632
Mitochondrial Uncoupler Prodrug of 2,4-Dinitrophenol, MP201, Prevents Neuronal Damage and Preserves Vision in Experimental Optic Neuritis
Abstract
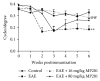
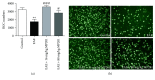
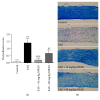
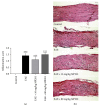
The ability of novel mitochondrial uncoupler prodrug of 2,4-dinitrophenol (DNP), MP201, to prevent neuronal damage and preserve visual function in an experimental autoimmune encephalomyelitis (EAE) model of optic neuritis was evaluated. Optic nerve inflammation, demyelination, and axonal loss are prominent features of optic neuritis, an inflammatory optic neuropathy often associated with the central nervous system demyelinating disease multiple sclerosis. Currently, optic neuritis is frequently treated with high-dose corticosteroids, but treatment fails to prevent permanent neuronal damage and associated vision changes that occur as optic neuritis resolves, thus suggesting that additional therapies are required. MP201 administered orally, once per day, attenuated visual dysfunction, preserved retinal ganglion cells (RGCs), and reduced RGC axonal loss and demyelination in the optic nerves of EAE mice, with limited effects on inflammation. The prominent mild mitochondrial uncoupling properties of MP201, with slow elimination of DNP, may contribute to the neuroprotective effect by modulating the entire mitochondria's physiology directly. Results suggest that MP201 is a potential novel treatment for optic neuritis.
Figures

Similar articles
-
Disease modifying mitochondrial uncouplers, MP101, and a slow release ProDrug, MP201, in models of Multiple Sclerosis.Neurochem Int. 2019 Dec;131:104561. doi: 10.1016/j.neuint.2019.104561. Epub 2019 Oct 1. Neurochem Int. 2019. PMID: 31585135
-
HE3286 reduces axonal loss and preserves retinal ganglion cell function in experimental optic neuritis.Invest Ophthalmol Vis Sci. 2014 Aug 19;55(9):5744-51. doi: 10.1167/iovs.14-14672. Invest Ophthalmol Vis Sci. 2014. PMID: 25139738 Free PMC article.
-
Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis.Biomolecules. 2022 Jun 14;12(6):830. doi: 10.3390/biom12060830. Biomolecules. 2022. PMID: 35740955 Free PMC article.
-
Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis.Oxid Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. Epub 2017 Feb 8. Oxid Med Cell Longev. 2017. PMID: 28270908 Free PMC article. Review.
-
Rodent Models of Optic Neuritis.Front Neurol. 2020 Nov 3;11:580951. doi: 10.3389/fneur.2020.580951. eCollection 2020. Front Neurol. 2020. PMID: 33224094 Free PMC article. Review.
Cited by
-
Matrine treatment reduces retinal ganglion cell apoptosis in experimental optic neuritis.Sci Rep. 2021 May 4;11(1):9520. doi: 10.1038/s41598-021-89086-7. Sci Rep. 2021. PMID: 33947942 Free PMC article.
-
Galectin-3 Plays a Role in Neuroinflammation in the Visual Pathway in Experimental Optic Neuritis.Cells. 2024 Mar 31;13(7):612. doi: 10.3390/cells13070612. Cells. 2024. PMID: 38607051 Free PMC article.
-
Current status of neuroprotective and neuroregenerative strategies in multiple sclerosis: A systematic review.Mult Scler. 2022 Jan;28(1):29-48. doi: 10.1177/13524585211008760. Epub 2021 Apr 19. Mult Scler. 2022. PMID: 33870797 Free PMC article.
-
The role of mitochondrial uncoupling in the regulation of mitostasis after traumatic brain injury.Neurochem Int. 2024 Mar;174:105680. doi: 10.1016/j.neuint.2024.105680. Epub 2024 Feb 3. Neurochem Int. 2024. PMID: 38311216
-
Activation of UCP2 by anethole trithione suppresses neuroinflammation after intracerebral hemorrhage.Acta Pharmacol Sin. 2022 Apr;43(4):811-828. doi: 10.1038/s41401-021-00698-1. Epub 2021 Jun 28. Acta Pharmacol Sin. 2022. PMID: 34183754 Free PMC article.
References
-
- Beck R. W., Gal R. L., Bhatti M. T. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. American Journal of Ophthalmology. 2004;137:77–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

