Cannabinoids and Vanilloids in Schizophrenia: Neurophysiological Evidence and Directions for Basic Research
- PMID: 28680405
- PMCID: PMC5478733
- DOI: 10.3389/fphar.2017.00399
Cannabinoids and Vanilloids in Schizophrenia: Neurophysiological Evidence and Directions for Basic Research
Abstract
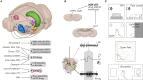
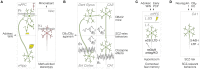
Much of our knowledge of the endocannabinoid system in schizophrenia comes from behavioral measures in rodents, like prepulse inhibition of the acoustic startle and open-field locomotion, which are commonly used along with neurochemical approaches or drug challenge designs. Such methods continue to map fundamental mechanisms of sensorimotor gating, hyperlocomotion, social interaction, and underlying monoaminergic, glutamatergic, and GABAergic disturbances. These strategies will require, however, a greater use of neurophysiological tools to better inform clinical research. In this sense, electrophysiology and viral vector-based circuit dissection, like optogenetics, can further elucidate how exogenous cannabinoids worsen (e.g., tetrahydrocannabinol, THC) or ameliorate (e.g., cannabidiol, CBD) schizophrenia symptoms, like hallucinations, delusions, and cognitive deficits. Also, recent studies point to a complex endocannabinoid-endovanilloid interplay, including the influence of anandamide (endogenous CB1 and TRPV1 agonist) on cognitive variables, such as aversive memory extinction. In fact, growing interest has been devoted to TRPV1 receptors as promising therapeutic targets. Here, these issues are reviewed with an emphasis on the neurophysiological evidence. First, we contextualize imaging and electrographic findings in humans. Then, we present a comprehensive review on rodent electrophysiology. Finally, we discuss how basic research will benefit from further combining psychopharmacological and neurophysiological tools.
Keywords: animal models; cannabinoids; electrophysiology; functional imaging; schizophrenia; vanilloids.
Figures

Similar articles
-
Effect of cannabidiol on endocannabinoid, glutamatergic and GABAergic signalling markers in male offspring of a maternal immune activation (poly I:C) model relevant to schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Dec 20;95:109666. doi: 10.1016/j.pnpbp.2019.109666. Epub 2019 Jun 14. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 31202911
-
Cannabidiol effects on prepulse inhibition in nonhuman primates.Rev Neurosci. 2018 Dec 19;30(1):95-105. doi: 10.1515/revneuro-2017-0101. Rev Neurosci. 2018. PMID: 29794254 Review.
-
The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents.CNS Neurol Disord Drug Targets. 2017;16(4):370-379. doi: 10.2174/1871527316666170223161908. CNS Neurol Disord Drug Targets. 2017. PMID: 28240187 Review.
-
A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice.Int J Neuropsychopharmacol. 2010 Aug;13(7):861-76. doi: 10.1017/S1461145709990605. Epub 2009 Sep 29. Int J Neuropsychopharmacol. 2010. PMID: 19785914
-
Cannabidiol improves behavioural and neurochemical deficits in adult female offspring of the maternal immune activation (poly I:C) model of neurodevelopmental disorders.Brain Behav Immun. 2019 Oct;81:574-587. doi: 10.1016/j.bbi.2019.07.018. Epub 2019 Jul 19. Brain Behav Immun. 2019. PMID: 31326506
Cited by
-
Multifunctional TRPV1 Ion Channels in Physiology and Pathology with Focus on the Brain, Vasculature, and Some Visceral Systems.Biomed Res Int. 2019 May 27;2019:5806321. doi: 10.1155/2019/5806321. eCollection 2019. Biomed Res Int. 2019. PMID: 31263706 Free PMC article. Review.
-
Treatments for Social Interaction Impairment in Animal Models of Schizophrenia: A Critical Review and Meta-analysis.Schizophr Bull. 2022 Nov 18;48(6):1179-1193. doi: 10.1093/schbul/sbac093. Schizophr Bull. 2022. PMID: 35925025 Free PMC article. Review.
-
Cannabis Use and Mental Illness: Understanding Circuit Dysfunction Through Preclinical Models.Front Psychiatry. 2021 Feb 5;12:597725. doi: 10.3389/fpsyt.2021.597725. eCollection 2021. Front Psychiatry. 2021. PMID: 33613338 Free PMC article. Review.
-
Long-term potentiation prevents ketamine-induced aberrant neurophysiological dynamics in the hippocampus-prefrontal cortex pathway in vivo.Sci Rep. 2020 Apr 28;10(1):7167. doi: 10.1038/s41598-020-63979-5. Sci Rep. 2020. PMID: 32346044 Free PMC article.
-
TRPV1 Channels in the Central Nervous System as Drug Targets.Pharmaceuticals (Basel). 2024 Jun 7;17(6):756. doi: 10.3390/ph17060756. Pharmaceuticals (Basel). 2024. PMID: 38931423 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

