An immunogenic personal neoantigen vaccine for patients with melanoma
- PMID: 28678778
- PMCID: PMC5577644
- DOI: 10.1038/nature22991
An immunogenic personal neoantigen vaccine for patients with melanoma
Erratum in
-
Corrigendum: An immunogenic personal neoantigen vaccine for patients with melanoma.Nature. 2018 Mar 14;555(7696):402. doi: 10.1038/nature25145. Nature. 2018. PMID: 29542692 Free PMC article.
Abstract
Effective anti-tumour immunity in humans has been associated with the presence of T cells directed at cancer neoantigens, a class of HLA-bound peptides that arise from tumour-specific mutations. They are highly immunogenic because they are not present in normal tissues and hence bypass central thymic tolerance. Although neoantigens were long-envisioned as optimal targets for an anti-tumour immune response, their systematic discovery and evaluation only became feasible with the recent availability of massively parallel sequencing for detection of all coding mutations within tumours, and of machine learning approaches to reliably predict those mutated peptides with high-affinity binding of autologous human leukocyte antigen (HLA) molecules. We hypothesized that vaccination with neoantigens can both expand pre-existing neoantigen-specific T-cell populations and induce a broader repertoire of new T-cell specificities in cancer patients, tipping the intra-tumoural balance in favour of enhanced tumour control. Here we demonstrate the feasibility, safety, and immunogenicity of a vaccine that targets up to 20 predicted personal tumour neoantigens. Vaccine-induced polyfunctional CD4+ and CD8+ T cells targeted 58 (60%) and 15 (16%) of the 97 unique neoantigens used across patients, respectively. These T cells discriminated mutated from wild-type antigens, and in some cases directly recognized autologous tumour. Of six vaccinated patients, four had no recurrence at 25 months after vaccination, while two with recurrent disease were subsequently treated with anti-PD-1 (anti-programmed cell death-1) therapy and experienced complete tumour regression, with expansion of the repertoire of neoantigen-specific T cells. These data provide a strong rationale for further development of this approach, alone and in combination with checkpoint blockade or other immunotherapies.
Conflict of interest statement
E.F.F is a founder and employee of Neon Therapeutics; N.H. and C.J.W. are founders of Neon Therapeutics and members of its scientific advisory board. P.A.O. has advised Neon Therapeutics. E.S.L. is a founder of Neon Therapeutics and a member of the Board of Directors. K.N. and I.J. are employees of CuriRx. Patent applications have been filed on aspects of the described work entitled as follows: Compositions and Methods for Personalized Neoplasia Vaccines (N.H., E.F.F., and C.J.W.), Methods for Identifying Tumor Specific Neo-Antigens (N.H. and C.J.W.), Formulations for Neoplasia Vaccines (E.F.F., K.N., and I.J.) and Combination Therapy for Neoantigen Vaccine (N.H., C.J.W., and E.F.F). S.J.R. receives research funding from Bristol-Myers Squibb, MedImmune and is on the scientific advisory board for Perkin Elmer Inc. The remaining authors declare no competing financial interests.
Figures
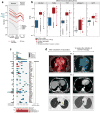
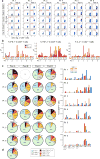

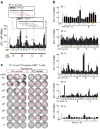


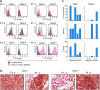
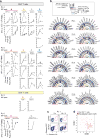



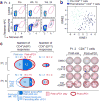
Comment in
-
Cancer: Precision T-cell therapy targets tumours.Nature. 2017 Jul 13;547(7662):165-167. doi: 10.1038/nature23093. Epub 2017 Jul 5. Nature. 2017. PMID: 28678783 No abstract available.
-
Tumour vaccines: Personal training by vaccination.Nat Rev Cancer. 2017 Jul 25;17(8):451. doi: 10.1038/nrc.2017.61. Nat Rev Cancer. 2017. PMID: 28740114 No abstract available.
-
Making It Personal: Neoantigen Vaccines in Metastatic Melanoma.Immunity. 2017 Aug 15;47(2):221-223. doi: 10.1016/j.immuni.2017.08.001. Immunity. 2017. PMID: 28813655
-
Neoantigen Vaccines Pass the Immunogenicity Test.Trends Mol Med. 2017 Oct;23(10):869-871. doi: 10.1016/j.molmed.2017.08.007. Epub 2017 Aug 31. Trends Mol Med. 2017. PMID: 28867556 Free PMC article.
Similar articles
-
Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens.Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23662-23670. doi: 10.1073/pnas.1906026116. Epub 2019 Nov 4. Proc Natl Acad Sci U S A. 2019. PMID: 31685621 Free PMC article.
-
A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer.Cell. 2020 Oct 15;183(2):347-362.e24. doi: 10.1016/j.cell.2020.08.053. Cell. 2020. PMID: 33064988 Clinical Trial.
-
HLA class I loss in metachronous metastases prevents continuous T cell recognition of mutated neoantigens in a human melanoma model.Oncotarget. 2017 Apr 25;8(17):28312-28327. doi: 10.18632/oncotarget.16048. Oncotarget. 2017. PMID: 28423700 Free PMC article.
-
Personalized neoantigen vaccines: A new approach to cancer immunotherapy.Bioorg Med Chem. 2018 Jun 1;26(10):2842-2849. doi: 10.1016/j.bmc.2017.10.021. Epub 2017 Oct 19. Bioorg Med Chem. 2018. PMID: 29111369 Review.
-
Neoantigen vaccine: an emerging tumor immunotherapy.Mol Cancer. 2019 Aug 23;18(1):128. doi: 10.1186/s12943-019-1055-6. Mol Cancer. 2019. PMID: 31443694 Free PMC article. Review.
Cited by
-
The MHC Motif Atlas: a database of MHC binding specificities and ligands.Nucleic Acids Res. 2023 Jan 6;51(D1):D428-D437. doi: 10.1093/nar/gkac965. Nucleic Acids Res. 2023. PMID: 36318236 Free PMC article.
-
A Systematic, Unbiased Mapping of CD8+ and CD4+ T Cell Epitopes in Yellow Fever Vaccinees.Front Immunol. 2020 Aug 31;11:1836. doi: 10.3389/fimmu.2020.01836. eCollection 2020. Front Immunol. 2020. PMID: 32983097 Free PMC article.
-
A randomised phase II trial of a trivalent ganglioside vaccine targeting GM2, GD2 and GD3 combined with immunological adjuvant OPT-821 versus OPT-821 alone in metastatic sarcoma patients rendered disease-free by surgery.Eur J Cancer. 2022 Nov;176:155-163. doi: 10.1016/j.ejca.2022.09.003. Epub 2022 Oct 8. Eur J Cancer. 2022. PMID: 36215947 Free PMC article. Clinical Trial.
-
Human TCR repertoire in cancer.Cancer Med. 2024 Sep;13(17):e70164. doi: 10.1002/cam4.70164. Cancer Med. 2024. PMID: 39240157 Free PMC article. Review.
-
Preclinical and Clinical Immunotherapeutic Strategies in Epithelial Ovarian Cancer.Cancers (Basel). 2020 Jul 2;12(7):1761. doi: 10.3390/cancers12071761. Cancers (Basel). 2020. PMID: 32630708 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

