Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation
- PMID: 28667163
- PMCID: PMC5531601
- DOI: 10.4049/jimmunol.1601569
Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation
Abstract
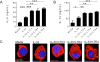
Asthma is a complex disease that is promoted by dysregulated immunity and the presence of many cytokine and lipid mediators. Despite this, there is a paucity of data demonstrating the combined effects of multiple mediators in asthma pathogenesis. Group 2 innate lymphoid cells (ILC2s) have recently been shown to play important roles in the initiation of allergic inflammation; however, it is unclear whether lipid mediators, such as cysteinyl leukotrienes (CysLTs), which are present in asthma, could further amplify the effects of IL-33 on ILC2 activation and lung inflammation. In this article, we show that airway challenges with the parent CysLT, leukotriene C4 (LTC4), given in combination with low-dose IL-33 to naive wild-type mice, led to synergistic increases in airway Th2 cytokines, eosinophilia, and peribronchial inflammation compared with IL-33 alone. Further, the numbers of proliferating and cytokine-producing lung ILC2s were increased after challenge with both LTC4 and IL-33. Levels of CysLT1R, CysLT2R, and candidate leukotriene E4 receptor P2Y12 mRNAs were increased in ILC2s. The synergistic effect of LTC4 with IL-33 was completely dependent upon CysLT1R, because CysLT1R-/- mice, but not CysLT2R-/- mice, had abrogated responses. Further, CysLTs directly potentiated IL-5 and IL-13 production from purified ILC2s stimulated with IL-33 and resulted in NFAT1 nuclear translocation. Finally, CysLT1R-/- mice had reduced lung eosinophils and ILC2 responses after exposure to the fungal allergen Alternaria alternata Thus, CysLT1R promotes LTC4- and Alternaria-induced ILC2 activation and lung inflammation. These findings suggest that multiple pathways likely exist in asthma to activate ILC2s and propagate inflammatory responses.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
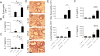

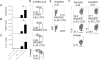
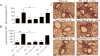
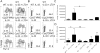
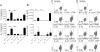
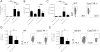
Similar articles
-
Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production.J Allergy Clin Immunol. 2013 Jul;132(1):205-13. doi: 10.1016/j.jaci.2013.03.048. Epub 2013 May 17. J Allergy Clin Immunol. 2013. PMID: 23688412 Free PMC article.
-
Type 2 Cysteinyl Leukotriene Receptors Drive IL-33-Dependent Type 2 Immunopathology and Aspirin Sensitivity.J Immunol. 2018 Feb 1;200(3):915-927. doi: 10.4049/jimmunol.1700603. Epub 2017 Dec 27. J Immunol. 2018. PMID: 29282304 Free PMC article.
-
Leukotriene D4 paradoxically limits LTC4-driven platelet activation and lung immunopathology.J Allergy Clin Immunol. 2021 Jul;148(1):195-208.e5. doi: 10.1016/j.jaci.2020.10.041. Epub 2020 Dec 4. J Allergy Clin Immunol. 2021. PMID: 33285161 Free PMC article.
-
Group 2 innate lymphoid cells in lung inflammation.Immunology. 2013 Nov;140(3):281-7. doi: 10.1111/imm.12153. Immunology. 2013. PMID: 23866009 Free PMC article. Review.
-
Type 2 innate lymphoid cells: at the cross-roads in allergic asthma.Semin Immunopathol. 2016 Jul;38(4):483-96. doi: 10.1007/s00281-016-0556-2. Epub 2016 Mar 10. Semin Immunopathol. 2016. PMID: 26965110 Free PMC article. Review.
Cited by
-
Innate Lymphoid Cells in Tissue Homeostasis and Disease Pathogenesis.Mol Cells. 2021 May 31;44(5):301-309. doi: 10.14348/molcells.2021.0053. Mol Cells. 2021. PMID: 33972473 Free PMC article. Review.
-
Prostaglandin E2 in NSAID-exacerbated respiratory disease: protection against cysteinyl leukotrienes and group 2 innate lymphoid cells.Curr Opin Allergy Clin Immunol. 2019 Feb;19(1):38-45. doi: 10.1097/ACI.0000000000000498. Curr Opin Allergy Clin Immunol. 2019. PMID: 30516547 Free PMC article. Review.
-
Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity.Immunity. 2019 Feb 19;50(2):505-519.e4. doi: 10.1016/j.immuni.2019.01.012. Epub 2019 Feb 12. Immunity. 2019. PMID: 30770247 Free PMC article.
-
Lower serum 15-HETE level predicts nasal ILC2 accumulation during COX-1 inhibition in AERD.J Allergy Clin Immunol. 2023 Nov;152(5):1330-1335.e1. doi: 10.1016/j.jaci.2023.06.028. Epub 2023 Aug 3. J Allergy Clin Immunol. 2023. PMID: 37543185 Free PMC article.
-
Innate lymphoid cells-key immune integrators of overall body homeostasis.Semin Immunopathol. 2018 Jul;40(4):319-330. doi: 10.1007/s00281-018-0684-y. Epub 2018 Jun 4. Semin Immunopathol. 2018. PMID: 29869059 Review.
References
-
- Weiss JW, Drazen JM, McFadden ER, Jr, Weller PF, Corey EJ, Lewis RA, Austen KF. Comparative bronchoconstrictor effects of histamine, leukotriene C, and leukotriene D in normal human volunteers. Trans Assoc Am Physicians. 1982;95:30–35. - PubMed
-
- Weiss JW, Drazen JM, Coles N, McFadden ER, Jr, Weller PF, Corey EJ, Lewis RA, Austen KF. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982;216:196–198. - PubMed
-
- Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet. 1993;341:989–990. - PubMed
-
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

