Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy
- PMID: 28662901
- PMCID: PMC5548264
- DOI: 10.1016/j.jconrel.2017.06.022
Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy
Abstract
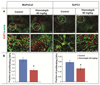
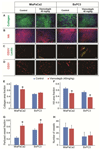
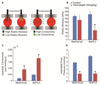
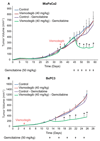
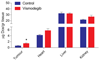
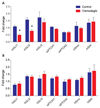
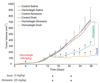
Targeting the rich extracellular matrix of desmoplastic tumors has been successfully shown to normalize collagen and hyaluronan levels and re-engineer intratumoral mechanical forces, improving tumor perfusion and chemotherapy. As far as targeting the abundant cancer-associated fibroblasts (CAFs) in desmoplastic tumors is concerned, while both pharmacologic inhibition of the sonic-hedgehog pathway and genetic depletion of fibroblasts have been employed in pancreatic cancers, the results between the two methods have been contradictory. In this study, we employed vismodegib to inhibit the sonic-hedgehog pathway with the aim to i) elucidate the mechanism of how CAFs depletion improves drug delivery, ii) extent and evaluate the potential use of sonic-hedgehog inhibitors to breast cancers, and iii) investigate whether sonic-hedgehog inhibition improves not only chemotherapy, but also the efficacy of the most commonly used breast cancer nanomedicines, namely Abraxane® and Doxil®. We found that treatment with vismodegib normalizes the tumor microenvironment by reducing the proliferative CAFs and in cases the levels of collagen and hyaluronan. These modulations re-engineered the solid and fluid stresses in the tumors, improving blood vessel functionality. As a result, the delivery and efficacy of chemotherapy was improved in two models of pancreatic cancer. Additionally, vismodegib treatment significantly improved the efficacy of both Abraxane and Doxil in xenograft breast tumors. Our results suggest the use of vismodegib, and sonic hedgehog inhibitors in general, to enhance cancer chemo- and nanotherapy.
Keywords: Breast cancer; Drug delivery; Nanomedicine; Pancreatic cancer; Re-engineering cancer; Tumor microenvironment; Tumor perfusion.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity.Theranostics. 2020 Jan 12;10(4):1910-1922. doi: 10.7150/thno.36936. eCollection 2020. Theranostics. 2020. PMID: 32042344 Free PMC article.
-
GDC-0449 improves the antitumor activity of nano-doxorubicin in pancreatic cancer in a fibroblast-enriched microenvironment.Sci Rep. 2017 Oct 17;7(1):13379. doi: 10.1038/s41598-017-13869-0. Sci Rep. 2017. PMID: 29042665 Free PMC article.
-
Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer.J Clin Oncol. 2015 Dec 20;33(36):4284-92. doi: 10.1200/JCO.2015.62.8719. Epub 2015 Nov 2. J Clin Oncol. 2015. PMID: 26527777 Free PMC article. Clinical Trial.
-
Nanomedicine applications in the treatment of breast cancer: current state of the art.Int J Nanomedicine. 2017 Aug 16;12:5879-5892. doi: 10.2147/IJN.S123437. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28860754 Free PMC article. Review.
-
Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration.Mol Pharm. 2020 Apr 6;17(4):1028-1048. doi: 10.1021/acs.molpharmaceut.0c00014. Epub 2020 Mar 16. Mol Pharm. 2020. PMID: 32150417 Review.
Cited by
-
Tumour vessel remodelling: new opportunities in cancer treatment.Vasc Biol. 2020 Jan 14;2(1):R35-R43. doi: 10.1530/VB-19-0032. eCollection 2020. Vasc Biol. 2020. PMID: 32923973 Free PMC article. Review.
-
In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate.Biomaterials. 2019 Oct;217:119296. doi: 10.1016/j.biomaterials.2019.119296. Epub 2019 Jun 21. Biomaterials. 2019. PMID: 31254934 Free PMC article.
-
Transforming growth factor-β modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion.Biochim Biophys Acta Gen Subj. 2018 Jul;1862(7):1537-1546. doi: 10.1016/j.bbagen.2018.02.009. Epub 2018 Mar 15. Biochim Biophys Acta Gen Subj. 2018. PMID: 29477748 Free PMC article.
-
Integrated Biophysical Characterization of Fibrillar Collagen-Based Hydrogels.ACS Biomater Sci Eng. 2020 Mar 9;6(3):1408-1417. doi: 10.1021/acsbiomaterials.9b01873. Epub 2020 Feb 5. ACS Biomater Sci Eng. 2020. PMID: 32292818 Free PMC article.
-
Vascular Normalization: A New Window Opened for Cancer Therapies.Front Oncol. 2021 Aug 12;11:719836. doi: 10.3389/fonc.2021.719836. eCollection 2021. Front Oncol. 2021. PMID: 34476218 Free PMC article. Review.
References
-
- Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanomedicine to tumors: Transport barriers and strategies. Annual Reviews Chemical and Biomolecular Engineering. 2011;2:281–298. - PubMed
-
- Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15101–15108. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

