Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants
- PMID: 28658720
- PMCID: PMC6481465
- DOI: 10.1002/14651858.CD007137.pub5
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants
Update in
-
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2020 Mar 31;3(3):CD007137. doi: 10.1002/14651858.CD007137.pub6. Cochrane Database Syst Rev. 2020. PMID: 32232984 Free PMC article.
Abstract
Background: Lactoferrin, a normal component of human colostrum and milk, can enhance host defenses and may be effective for prevention of sepsis and necrotizing enterocolitis (NEC) in preterm neonates.
Objectives: Primary objective 1. To assess the safety and effectiveness of lactoferrin supplementation to enteral feeds for prevention of sepsis and NEC in preterm neonates Secondary objectives 1. To determine the effects of lactoferrin supplementation to enteral feeds to prevent neonatal sepsis and/or NEC on duration of positive-pressure ventilation, development of chronic lung disease (CLD) or periventricular leukomalacia (PVL), length of hospital stay to discharge among survivors, and adverse neurological outcomes at two years of age or later2. To determine the adverse effects of lactoferrin supplementation for prophylaxis of neonatal sepsis and/or NECWhen data were available, we analyzed the following subgroups.1. Gestational age < 32 weeks and 32 to 36 weeks2. Birth weight < 1000 g (extremely low birth weight (ELBW) infants) and birth weight < 1500 g (very low birth weight (VLBW) infants)3. Type of feeding: breast milk versus formula milk SEARCH METHODS: We used the search strategy of the Cochrane Neonatal Review Group (CNRG) to update our search in December 2016. We searched the databases Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PREMEDLINE, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), as well as trial registries and conference proceedings.
Selection criteria: Randomized controlled trials (RCTs) evaluating oral lactoferrin at any dose or duration to prevent sepsis or NEC in preterm neonates.
Data collection and analysis: Review authors used standard methods of the CNRG.
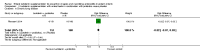
Main results: This review includes six RCTs. Trial results show that lactoferrin supplementation to enteral feeds decreased late-onset sepsis (typical risk ratio (RR) 0.59, 95% confidence interval (CI) 0.40 to 0.87; typical risk difference (RD) -0.06, 95% CI -0.10 to -0.02; number needed to treat for an additional beneficial outcome (NNTB) 17, 95% CI 10 to 50; six trials, 886 participants; low-quality evidence) and NEC stage II or III (typical RR 0.40, 95% CI 0.18 to 0.86; typical RD -0.04, 95% CI -0.06 to -0.01; NNTB 25, 95% CI 17 to 100; four studies, 750 participants; low-quality evidence). Lactoferrin supplementation did not have an effect on "all-cause mortality" (typical RR 0.65, 95% CI 0.37 to 1.11; typical RD -0.02, 95% CI -0.05 to 0; six studies, 1041 participants; low-quality evidence).Lactoferrin supplementation to enteral feeds with probiotics decreased late-onset sepsis (RR 0.27, 95% CI 0.12 to 0.60; RD -0.13, 95% CI -0.19 to -0.06; NNTB 8, 95% CI 5 to 17; one study, 321 participants; low-quality evidence) and NEC stage II or III (RR 0.04, 95% CI 0.00 to 0.62; RD -0.05, 95% CI -0.08 to -0.03; NNTB 20, 95% CI 12.5 to 33.3; one study, 496 participants; low-quality evidence), but not "all-cause mortality" (low-quality evidence).Lactoferrin supplementation to enteral feeds with or without probiotics decreased bacterial and fungal sepsis but not CLD or length of hospital stay (low-quality evidence). Investigators reported no adverse effects and did not evaluate long-term neurological outcomes and PVL.
Authors' conclusions: Evidence of low quality suggests that lactoferrin supplementation to enteral feeds with or without probiotics decreases late-onset sepsis and NEC stage II or III in preterm infants without adverse effects. Completed ongoing trials will provide data from more than 6000 preterm neonates, which may enhance the quality of the evidence. Clarification regarding optimal dosing regimens, types of lactoferrin (human or bovine), and long-term outcomes is needed.
Conflict of interest statement
Agennix, Inc., donated human recombinant lactoferrin for Dr Pammi's laboratory research from 2006 through 2009.
Figures





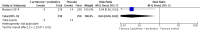
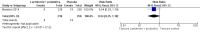
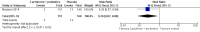



















Update of
-
Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2015 Feb 20;(2):CD007137. doi: 10.1002/14651858.CD007137.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Jun 28;6:CD007137. doi: 10.1002/14651858.CD007137.pub5. PMID: 25699678 Updated. Review.
Similar articles
-
Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2020 Mar 31;3(3):CD007137. doi: 10.1002/14651858.CD007137.pub6. Cochrane Database Syst Rev. 2020. PMID: 32232984 Free PMC article.
-
Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2015 Feb 20;(2):CD007137. doi: 10.1002/14651858.CD007137.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Jun 28;6:CD007137. doi: 10.1002/14651858.CD007137.pub5. PMID: 25699678 Updated. Review.
-
Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2010 May 12;(5):CD007137. doi: 10.1002/14651858.CD007137.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2011 Oct 05;(10):CD007137. doi: 10.1002/14651858.CD007137.pub3. PMID: 20464748 Updated. Review.
-
Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007137. doi: 10.1002/14651858.CD007137.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Feb 20;(2):CD007137. doi: 10.1002/14651858.CD007137.pub4. PMID: 21975765 Updated. Review.
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD000366. doi: 10.1002/14651858.CD000366.pub4. Cochrane Database Syst Rev. 2019. PMID: 31283839 Free PMC article.
Cited by
-
Human Breast Milk: The Key Role in the Maturation of Immune, Gastrointestinal and Central Nervous Systems: A Narrative Review.Diagnostics (Basel). 2022 Sep 12;12(9):2208. doi: 10.3390/diagnostics12092208. Diagnostics (Basel). 2022. PMID: 36140609 Free PMC article. Review.
-
A critical analysis of risk factors for necrotizing enterocolitis.Semin Fetal Neonatal Med. 2018 Dec;23(6):374-379. doi: 10.1016/j.siny.2018.07.005. Epub 2018 Aug 1. Semin Fetal Neonatal Med. 2018. PMID: 30115546 Free PMC article. Review.
-
Nutrition for Preterm Infants: 75 Years of History.Ann Nutr Metab. 2018;72 Suppl 3(Suppl 3):25-31. doi: 10.1159/000487378. Epub 2018 Apr 10. Ann Nutr Metab. 2018. PMID: 29635225 Free PMC article.
-
Lactoferrin and Its Potential Impact for the Relief of Pain: A Preclinical Approach.Pharmaceuticals (Basel). 2021 Aug 28;14(9):868. doi: 10.3390/ph14090868. Pharmaceuticals (Basel). 2021. PMID: 34577568 Free PMC article. Review.
-
Safety and efficacy of probiotic administration to preterm infants: ten common questions.Pediatr Res. 2020 Aug;88(Suppl 1):48-55. doi: 10.1038/s41390-020-1080-6. Pediatr Res. 2020. PMID: 32855513 Free PMC article. Review.
References
References to studies included in this review
-
- Akin IM, Atasay B, Dogu F, Okulu E, Arsan S, Karatas HD, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T‐regulatory cells. American Journal of Perinatology 2014;31(12):1111‐20. [DOI: 10.1055/s-0034-1371704; PUBMED: 24839144] - DOI - PubMed
-
- Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very‐low‐birth‐weight neonates: a randomized clinical trial. Early Human Development 2014;90 Suppl 1:S60‐5. [DOI: 10.1016/S0378-3782(14)70020-9; PUBMED: 24709463] - DOI - PubMed
- Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, The Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late‐onset sepsis in very low‐birth‐weight neonates: a randomized trial. JAMA 2009;302(13):1421‐8. [PUBMED: DOI: 10.1001/jama.2009.1403; PubMed: 19809023]] - PubMed
- Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, The Italian Society of Neonatology. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 2012;129(1):116‐23. [PUBMED: DOI: 10.1542/peds.2011‐0279; PubMed: 22184648]] - PubMed
-
- Ochoa TJ, Zegarra J, Cam L, Llanos R, Pezo A, Cruz K, et al. NEOLACTO Research Group. Randomized controlled trial of lactoferrin for prevention of sepsis in Peruvian neonates less than 2500 g. Pediatric Infectious Disease Journal 2015;34(6):571‐6. [DOI: 10.1097/INF.0000000000000593; NCT01264536] - DOI - PMC - PubMed
References to studies excluded from this review
-
- King JC Jr, Cummings GE, Guo N, Trivedi L, Readmond BX, Keane V, et al. A double‐blind, placebo‐controlled, pilot study of bovine lactoferrin supplementation in bottle‐fed infants. Journal of Pediatric Gastroenterology and Nutrition 2007;44(2):245‐51. [DOI: 10.1097/01.mpg.0000243435.54958.68; PUBMED: 17255839] - DOI - PubMed
-
- Meyer MP, Alexander T. Reduced rates of necrotizing enterocolitis following routine supplementation with Lactobacillus GG and lactoferrin: an observational study. Poster Session: Neonatology: Neonatal GI Physiology & NEC: Probiotics, Antibiotics and Microbiome. Pediatric Academic Societies Meeting, May 16, 2016, Baltimore Convention Center. Baltimore, Maryland, 2016. [E‐PAS2016:4159.485]
References to studies awaiting assessment
-
- ISRCTN71737811. Effect of prebiotic or lactoferrin supplementation in formula on the gut flora of preterm infants. www.controlled‐trials.com/ISCRTN71737811 (first received 05 September 2008). [DOI: 10.1186/ISRCTN71737811] - DOI
-
- NCT01172236. Supplementation with lactoferrin in preterm newborns (lactoprenew). clinicaltrials.gov/show/NCT01172236 (first received 28 July 2010).
-
- NCT02959229. Early versus late lactoferrin in prevention of neonatal sepsis [Systematic randomized, single blinded, placebo‐controlled trial of early versus late lactoferrin in prevention of neonatal sepsis]. clinicaltrials.gov/show/NCT02959229 (first received 27 October 2016).
References to ongoing studies
-
- ACTRN12611000247976. Lactoferrin infant feeding trial (LIFT) to prevent sepsis and death in preterm infants [Does lactoferrin improve survival free from morbidity in very low birth weight infants? Lactoferrin Infant Feeding Trial: a randomised controlled trial]. www.anzctr.org.au/ACTRN12611000247976 (first received 03 March 2011).
-
- ISRCTN88261002. Enteral lactoferrin In neonates. www.controlled‐trials.com/ISRCTN88261002 (first received 05 June 2013). [DOI: 10.1186/ISRCTN88261002] - DOI
-
- NCT01525316. Lactoferrin for prevention of sepsis in infants (NEOLACTO). clinicaltrials.gov/show/NCT01525316 (first received 30 January 2012).
-
- NCT01821989. Oral lactoferrin supplementation for prevention of sepsis in preterm neonate. clinicaltrials.gov/show/NCT01821989 (first received 27 March 2013).
Additional references
-
- Barboza M, Pinzon J, Wickramasinghe S, Froehlich J, Moeller I, Smilowitz J, et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria‐host interactions. Molecular Cell Proteomics 2012;11(6):M111.015248. [DOI: 10.1074/mcp.M111.015248; PUBMED: 22261723] - DOI - PMC - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

