Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia-mediated neurodegeneration
- PMID: 28655763
- PMCID: PMC5555200
- DOI: 10.1074/jbc.M117.784983
Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia-mediated neurodegeneration
Abstract
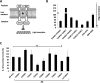
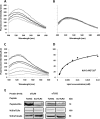
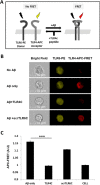
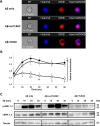
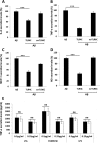
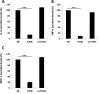
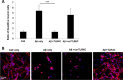
Recently, a single study revealed a new complex composed of Toll-like receptor 4 (TLR4), TLR6, and CD36 induced by fibrillary Aβ peptides, the hallmark of Alzheimer's disease. Unlike TLRs located on the plasma membrane that dimerize on the membrane after ligand binding to their extracellular domain, the TLR4-TLR6-CD36 complex assembly has been suggested to be induced by intracellular signals from CD36, similar to integrin inside-out signaling. However, the assembly site of TLR4-TLR6-CD36 and the domains participating in Aβ-induced signaling is still unknown. By interfering with TLR4-TLR6 dimerization using a TLR4-derived peptide, we show that receptor assembly is abrogated within the plasma membrane. Furthermore, we reveal that the transmembrane domains of TLR4 and TLR6 have an essential role in receptor dimerization and activation. Inhibition of TLR4-TLR6 assembly was associated with reduced secretion of proinflammatory mediators from microglia cells, ultimately rescuing neurons from death. Our findings support TLR4-TLR6 dimerization induced by Aβ. Moreover, we shed new light on TLR4-TLR6 assembly and localization and show the potential of inhibiting TLR4-TLR6 dimerization as a treatment of Alzheimer's disease.
Keywords: Alzheimer disease; CD36; TLR4; TLR6; amyloid-β; inflammation; microglia; neurodegenerative disease; neuroinflammation; toll-like receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer.Nat Immunol. 2010 Feb;11(2):155-61. doi: 10.1038/ni.1836. Epub 2009 Dec 27. Nat Immunol. 2010. PMID: 20037584 Free PMC article.
-
IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia.J Immunol. 2008 Nov 1;181(9):6503-13. doi: 10.4049/jimmunol.181.9.6503. J Immunol. 2008. PMID: 18941241
-
Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease.Cell Physiol Biochem. 2007;20(6):947-56. doi: 10.1159/000110455. Cell Physiol Biochem. 2007. PMID: 17982277
-
Microglial Aβ receptors in Alzheimer's disease.Cell Mol Neurobiol. 2015 Jan;35(1):71-83. doi: 10.1007/s10571-014-0101-6. Epub 2014 Aug 23. Cell Mol Neurobiol. 2015. PMID: 25149075 Review.
-
Role of Toll Like Receptor 4 in Alzheimer's Disease.Front Immunol. 2020 Aug 26;11:1588. doi: 10.3389/fimmu.2020.01588. eCollection 2020. Front Immunol. 2020. PMID: 32983082 Free PMC article. Review.
Cited by
-
Computational Deciphering of the Role of S100A8 and S100A9 Proteins and Their Changes in the Structure Assembly Influences Their Interaction with TLR4, RAGE, and CD36.Protein J. 2024 Apr;43(2):243-258. doi: 10.1007/s10930-024-10186-0. Epub 2024 Mar 2. Protein J. 2024. PMID: 38431537
-
Distinctive Toll-like Receptors Gene Expression and Glial Response in Different Brain Regions of Natural Scrapie.Int J Mol Sci. 2022 Mar 25;23(7):3579. doi: 10.3390/ijms23073579. Int J Mol Sci. 2022. PMID: 35408945 Free PMC article.
-
Lipid rafts in glial cells: role in neuroinflammation and pain processing.J Lipid Res. 2020 May;61(5):655-666. doi: 10.1194/jlr.TR119000468. Epub 2019 Dec 20. J Lipid Res. 2020. PMID: 31862695 Free PMC article. Review.
-
Glial Cell-Mediated Neuroinflammation in Alzheimer's Disease.Int J Mol Sci. 2022 Sep 12;23(18):10572. doi: 10.3390/ijms231810572. Int J Mol Sci. 2022. PMID: 36142483 Free PMC article. Review.
-
Identification of the Association Between Toll-Like Receptors and T-Cell Activation in Takayasu's Arteritis.Front Immunol. 2022 Jan 20;12:792901. doi: 10.3389/fimmu.2021.792901. eCollection 2021. Front Immunol. 2022. PMID: 35126357 Free PMC article.
References
-
- Vadillo E., and Pelayo R. (2012) Toll-like receptors in development and function of the hematopoietic system. Rev. Invest. Clin. 64, 461–476 - PubMed
-
- Akira S., and Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 - PubMed
-
- Kaisho T., and Akira S. (2006) Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 117, 979–987 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

