Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation
- PMID: 28652735
- PMCID: PMC5473603
- DOI: 10.2147/IJN.S136281
Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation
Abstract
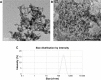
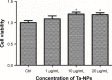
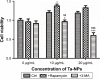
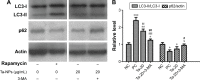
Porous tantalum (Ta) implants are highly corrosion resistant and biocompatible, and they possess significantly better initial stability than that of conventional titanium (Ti) implants. During loading wear, Ta nanoparticles (Ta-NPs) that were deposited on the surface of a porous Ta implant are inevitably released and come into direct contact with peri-implant osteoblasts. The wear debris may influence cell behavior and implant stabilization. However, the interaction of Ta-NPs with osteoblasts has not been clearly investigated. This study aimed to investigate the effect of Ta-NPs on cell proliferation and their underlying mechanism. The Cell Counting Kit-8 (CCK-8) assay was used to measure the cell viability of MC3T3-E1 mouse osteoblasts and showed that Ta-NP treatment could increase cell viability. Then, confocal microscopy, Western blotting, and transmission electron microscopy were used to confirm the autophagy induced by Ta-NPs, and evidence of autophagy induction was observed as positive LC3 puncta, high-LC3-II expression, and autophagic vesicle ultrastructures. The CCK-8 assay revealed that the cell viability was further increased and decreased by the application of an autophagy inducer and inhibitor, respectively. In addition, pre-treatment with autophagy inhibitor 3-methyladenine (3-MA) inhibited the Ta-NP-induced autophagy. These results indicate that the Ta-NPs can promote cell proliferation, that an autophagy inducer can further strengthen this effect and that an autophagy inhibitor can weaken this effect. In conclusion, autophagy was involved in Ta-NP-induced cell proliferation and had a promoting effect.
Keywords: autophagy; osteoblast; proliferation; tantalum nanoparticles.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Assessment of tantalum nanoparticle-induced MC3T3-E1 proliferation and underlying mechanisms.J Mater Sci Mater Med. 2021 Oct 23;32(11):133. doi: 10.1007/s10856-021-06606-7. J Mater Sci Mater Med. 2021. PMID: 34689241 Free PMC article.
-
Cytotoxicity, Oxidative Stress, and Autophagy Effects of Tantalum Nanoparticles on MC3T3-E1 Mouse Osteoblasts.J Nanosci Nanotechnol. 2020 Mar 1;20(3):1417-1424. doi: 10.1166/jnn.2020.17158. J Nanosci Nanotechnol. 2020. PMID: 31492302
-
Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties.Acta Biomater. 2010 Aug;6(8):3349-59. doi: 10.1016/j.actbio.2010.01.046. Epub 2010 Feb 2. Acta Biomater. 2010. PMID: 20132912 Free PMC article.
-
The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology.Mater Sci Eng C Mater Biol Appl. 2015 Apr;49:323-329. doi: 10.1016/j.msec.2015.01.007. Epub 2015 Jan 6. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25686956 Review.
-
The Clinical Application of Porous Tantalum and Its New Development for Bone Tissue Engineering.Materials (Basel). 2021 May 18;14(10):2647. doi: 10.3390/ma14102647. Materials (Basel). 2021. PMID: 34070153 Free PMC article. Review.
Cited by
-
Assessment of tantalum nanoparticle-induced MC3T3-E1 proliferation and underlying mechanisms.J Mater Sci Mater Med. 2021 Oct 23;32(11):133. doi: 10.1007/s10856-021-06606-7. J Mater Sci Mater Med. 2021. PMID: 34689241 Free PMC article.
-
Tantalum Particles Induced Cytotoxic and Inflammatory Effects in Human Monocytes.Biomed Res Int. 2021 Jan 29;2021:6658498. doi: 10.1155/2021/6658498. eCollection 2021. Biomed Res Int. 2021. PMID: 33564679 Free PMC article.
-
Implantable PEKK/tantalum microparticles composite with improved surface performances for regulating cell behaviors, promoting bone formation and osseointegration.Bioact Mater. 2020 Oct 8;6(4):928-940. doi: 10.1016/j.bioactmat.2020.09.021. eCollection 2021 Apr. Bioact Mater. 2020. PMID: 33102936 Free PMC article.
-
[Sericin regulates proliferation of human gastric cancer MKN45 cells through autophagic pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2018 Feb 20;38(2):148-154. doi: 10.3969/j.issn.1673-4254.2018.02.05. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 29502052 Free PMC article. Chinese.
-
Roles of inflammatory cell infiltrate in periprosthetic osteolysis.Front Immunol. 2023 Dec 1;14:1310262. doi: 10.3389/fimmu.2023.1310262. eCollection 2023. Front Immunol. 2023. PMID: 38106424 Free PMC article. Review.
References
-
- Zhu Y, Gu Y, Qiao S, Zhou L, Shi J, Lai H. Bacterial and mammalian cells adhesion to tantalum-decorated micro-/nano-structured titanium. J Biomed Mater Res A. 2017;105(3):871–878. - PubMed
-
- Zhang D, Wong CS, Wen C, Li Y. Cellular responses of osteoblast-like cells to 17 elemental metals. J Biomed Mater Res A. 2017;105(1):148–158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

