Interrogating Detergent Desolvation of Nanopore-Forming Proteins by Fluorescence Polarization Spectroscopy
- PMID: 28650154
- PMCID: PMC5558884
- DOI: 10.1021/acs.analchem.7b01339
Interrogating Detergent Desolvation of Nanopore-Forming Proteins by Fluorescence Polarization Spectroscopy
Abstract
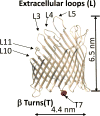
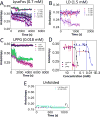
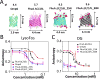
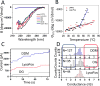
Understanding how membrane proteins interact with detergents is of fundamental and practical significance in structural and chemical biology as well as in nanobiotechnology. Current methods for inspecting protein-detergent complex (PDC) interfaces require high concentrations of protein and are of low throughput. Here, we describe a scalable, spectroscopic approach that uses nanomolar protein concentrations in native solutions. This approach, which is based on steady-state fluorescence polarization (FP) spectroscopy, kinetically resolves the dissociation of detergents from membrane proteins and protein unfolding. For satisfactorily solubilizing detergents, at concentrations much greater than the critical micelle concentration (CMC), the fluorescence anisotropy was independent of detergent concentration. In contrast, at detergent concentrations comparable with or below the CMC, the anisotropy readout underwent a time-dependent decrease, showing a specific and sensitive protein unfolding signature. Functionally reconstituted membrane proteins into a bilayer membrane confirmed predictions made by these FP-based determinations with respect to varying refolding conditions. From a practical point of view, this 96-well analytical approach will facilitate a massively parallel assessment of the PDC interfacial interactions under a fairly broad range of micellar and environmental conditions. We expect that these studies will potentially accelerate research in membrane proteins pertaining to their extraction, solubilization, stabilization, and crystallization, as well as reconstitution into bilayer membranes.
Figures
Similar articles
-
Detergent Desorption of Membrane Proteins Exhibits Two Kinetic Phases.J Phys Chem Lett. 2018 Apr 19;9(8):1913-1919. doi: 10.1021/acs.jpclett.8b00549. Epub 2018 Apr 2. J Phys Chem Lett. 2018. PMID: 29595981 Free PMC article.
-
High-Throughput Screening of Protein-Detergent Complexes Using Fluorescence Polarization Spectroscopy.Curr Protoc Protein Sci. 2019 Sep;97(1):e96. doi: 10.1002/cpps.96. Curr Protoc Protein Sci. 2019. PMID: 31517448 Free PMC article.
-
Kinetics of Membrane Protein-Detergent Interactions Depend on Protein Electrostatics.J Phys Chem B. 2018 Oct 18;122(41):9471-9481. doi: 10.1021/acs.jpcb.8b07889. Epub 2018 Oct 5. J Phys Chem B. 2018. PMID: 30251852 Free PMC article.
-
Interaction of membrane proteins and lipids with solubilizing detergents.Biochim Biophys Acta. 2000 Nov 23;1508(1-2):86-111. doi: 10.1016/s0304-4157(00)00010-1. Biochim Biophys Acta. 2000. PMID: 11090820 Review.
-
The mechanism of detergent solubilization of lipid bilayers.Biophys J. 2013 Jul 16;105(2):289-99. doi: 10.1016/j.bpj.2013.06.007. Biophys J. 2013. PMID: 23870250 Free PMC article. Review.
Cited by
-
Fluorescence Polarization-Based Bioassays: New Horizons.Sensors (Basel). 2020 Dec 12;20(24):7132. doi: 10.3390/s20247132. Sensors (Basel). 2020. PMID: 33322750 Free PMC article. Review.
-
Convergent Alterations of a Protein Hub Produce Divergent Effects within a Binding Site.ACS Chem Biol. 2022 Jun 17;17(6):1586-1597. doi: 10.1021/acschembio.2c00273. Epub 2022 May 25. ACS Chem Biol. 2022. PMID: 35613319 Free PMC article.
-
Detergent Desorption of Membrane Proteins Exhibits Two Kinetic Phases.J Phys Chem Lett. 2018 Apr 19;9(8):1913-1919. doi: 10.1021/acs.jpclett.8b00549. Epub 2018 Apr 2. J Phys Chem Lett. 2018. PMID: 29595981 Free PMC article.
-
Quantification of Membrane Protein-Detergent Complex Interactions.J Phys Chem B. 2017 Nov 9;121(44):10228-10241. doi: 10.1021/acs.jpcb.7b08045. Epub 2017 Oct 31. J Phys Chem B. 2017. PMID: 29035562 Free PMC article.
-
Real-Time Measurement of a Weak Interaction of a Transcription Factor Motif with a Protein Hub at Single-Molecule Precision.ACS Nano. 2024 Jul 25;18(31):20468-81. doi: 10.1021/acsnano.4c04857. Online ahead of print. ACS Nano. 2024. PMID: 39049818 Free PMC article.
References
-
- Pollock NL, Satriano L, Zegarra-Moran O, Ford RC, Moran O. J. Struct. Biol. 2016;194:102–111. - PubMed
-
- Grosse W, Psakis G, Mertins B, Reiss P, Windisch D, Brademann F, Burck J, Ulrich A, Koert U, Essen LO. Biochemistry. 2014;53:4826–4838. - PubMed
-
- Tulumello DV, Deber CM. Biochemistry. 2009;48:12096–12103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

