Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness
- PMID: 28649800
- PMCID: PMC5496497
- DOI: 10.1002/1878-0261.12096
Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness
Abstract
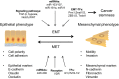
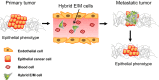
Epithelial-mesenchymal transition (EMT) is an important process in embryonic development, fibrosis, and cancer metastasis. During cancer progression, the activation of EMT permits cancer cells to acquire migratory, invasive, and stem-like properties. A growing body of evidence supports the critical link between EMT and cancer stemness. However, contradictory results have indicated that the inhibition of EMT also promotes cancer stemness, and that mesenchymal-epithelial transition, the reverse process of EMT, is associated with the tumor-initiating ability required for metastatic colonization. The concept of 'intermediate-state EMT' provides a possible explanation for this conflicting evidence. In addition, recent studies have indicated that the appearance of 'hybrid' epithelial-mesenchymal cells is favorable for the establishment of metastasis. In summary, dynamic changes or plasticity between the epithelial and the mesenchymal states rather than a fixed phenotype is more likely to occur in tumors in the clinical setting. Further studies aimed at validating and consolidating the concept of intermediate-state EMT and hybrid tumors are needed for the establishment of a comprehensive profile of cancer metastasis.
Keywords: epithelial-mesenchymal transition; metastasis; plasticity; stemness.
© 2017 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures
Similar articles
-
Hybrid Epithelial/Mesenchymal State in Cancer Metastasis: Clinical Significance and Regulatory Mechanisms.Cells. 2020 Mar 4;9(3):623. doi: 10.3390/cells9030623. Cells. 2020. PMID: 32143517 Free PMC article. Review.
-
Immunological Consequences of Epithelial-Mesenchymal Transition in Tumor Progression.J Immunol. 2016 Aug 1;197(3):691-8. doi: 10.4049/jimmunol.1600458. J Immunol. 2016. PMID: 27431984 Free PMC article. Review.
-
Cell stemness, epithelial-to-mesenchymal transition, and immunoevasion: Intertwined aspects in cancer metastasis.Semin Cancer Biol. 2020 Feb;60:181-190. doi: 10.1016/j.semcancer.2019.08.015. Epub 2019 Aug 15. Semin Cancer Biol. 2020. PMID: 31422157 Review.
-
Epithelial, mesenchymal and hybrid epithelial/mesenchymal phenotypes and their clinical relevance in cancer metastasis.Expert Rev Mol Med. 2017 Mar 21;19:e3. doi: 10.1017/erm.2017.6. Expert Rev Mol Med. 2017. PMID: 28322181 Review.
-
The EMT universe: space between cancer cell dissemination and metastasis initiation.Crit Rev Oncog. 2014;19(5):349-61. doi: 10.1615/critrevoncog.2014011802. Crit Rev Oncog. 2014. PMID: 25404150 Review.
Cited by
-
Interleukin-1β and Cancer.Cancers (Basel). 2020 Jul 4;12(7):1791. doi: 10.3390/cancers12071791. Cancers (Basel). 2020. PMID: 32635472 Free PMC article. Review.
-
Interfering HMGB3 release from cancer-associated fibroblasts by miR-200b represses chemoresistance and epithelial-mesenchymal transition of gastric cancer cells.J Gastrointest Oncol. 2022 Oct;13(5):2197-2218. doi: 10.21037/jgo-22-723. J Gastrointest Oncol. 2022. PMID: 36388689 Free PMC article.
-
The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner.Nat Commun. 2018 Aug 16;9(1):3279. doi: 10.1038/s41467-018-05793-2. Nat Commun. 2018. PMID: 30115931 Free PMC article.
-
Reduced Zeb1 Expression in Prostate Cancer Cells Leads to an Aggressive Partial-EMT Phenotype Associated with Altered Global Methylation Patterns.Int J Mol Sci. 2021 Nov 27;22(23):12840. doi: 10.3390/ijms222312840. Int J Mol Sci. 2021. PMID: 34884649 Free PMC article.
-
Natural compounds: Wnt pathway inhibitors with therapeutic potential in lung cancer.Front Pharmacol. 2023 Sep 28;14:1250893. doi: 10.3389/fphar.2023.1250893. eCollection 2023. Front Pharmacol. 2023. PMID: 37841927 Free PMC article. Review.
References
-
- Ambros V (2004) The functions of animal microRNAs. Nature 431, 350–355. - PubMed
-
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S et al (2008) Induction of EMT by twist proteins as a collateral effect of tumor‐promoting inactivation of premature senescence. Cancer Cell 14, 79–89. - PubMed
-
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ and Garcia‐Blanco MA (2011) Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 9, 997–1007. - PMC - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

