HOXB7 accelerates the malignant progression of hepatocellular carcinoma by promoting stemness and epithelial-mesenchymal transition
- PMID: 28646927
- PMCID: PMC5483250
- DOI: 10.1186/s13046-017-0559-4
HOXB7 accelerates the malignant progression of hepatocellular carcinoma by promoting stemness and epithelial-mesenchymal transition
Abstract
Background: Homeobox B7 (HOXB7) has been identified associated with poor prognosis of hepatocellular carcinoma (HCC). However, the specific mechanism by which HOXB7 promotes the malignant progression of HCC remains to be determined.
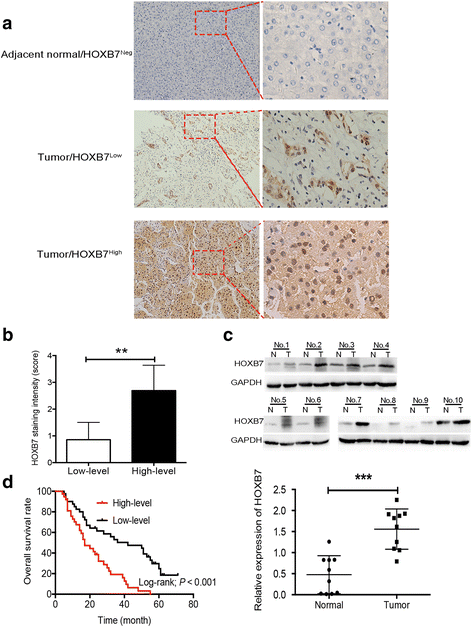
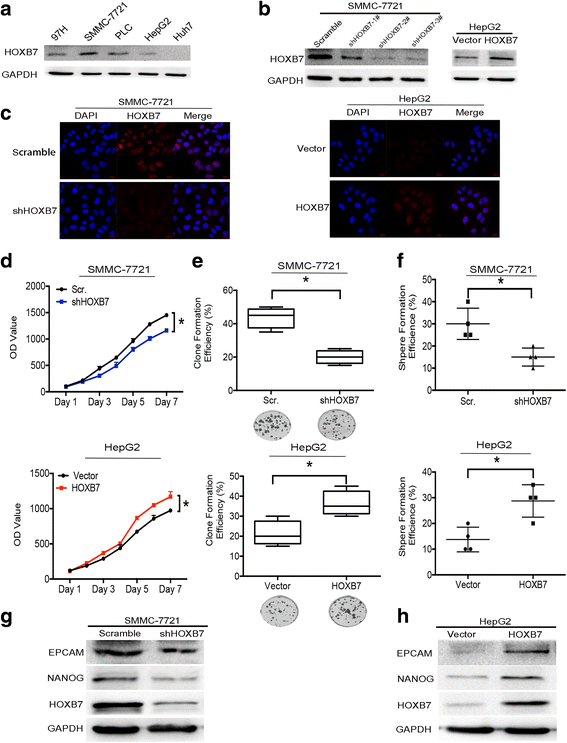
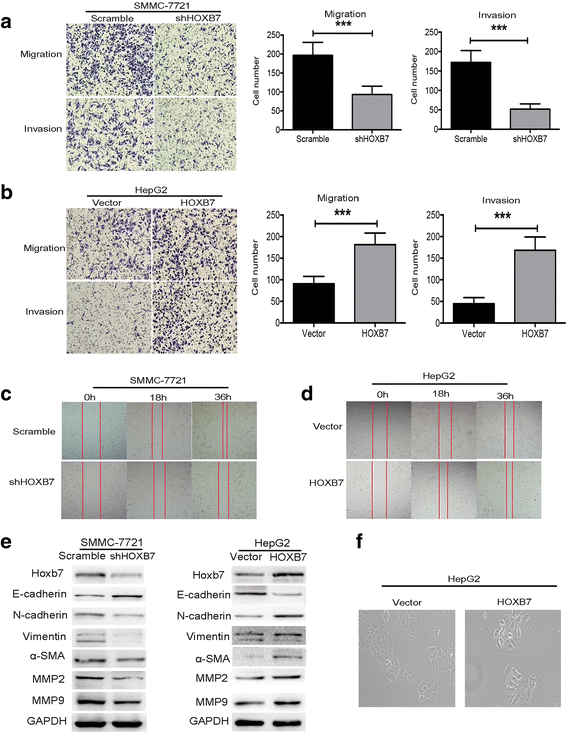
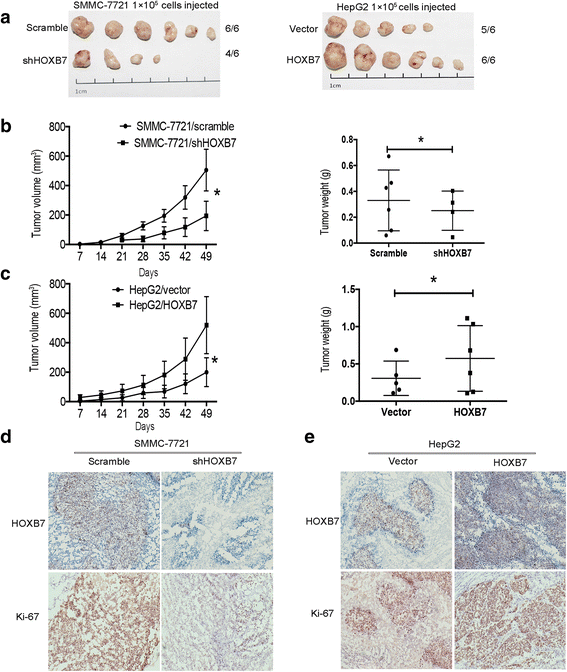
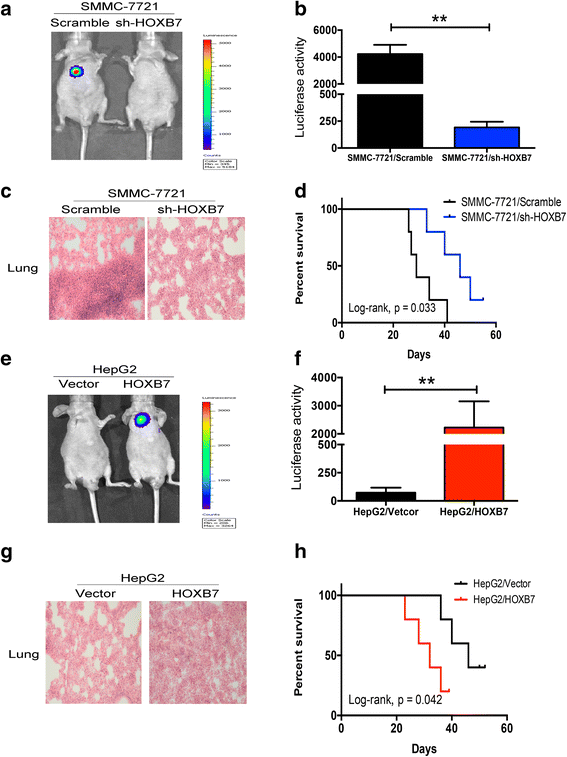
Methods: Immunohistochemistry (IHC) was used to detect the expression level of HOXB7 in 77-paired HCC tissue samples, and the correlation between HOXB7 and HCC prognosis was assessed. The location of HOXB7 was confirmed by immunofluorescence. Cell Titer-Blue assay was used to assess the proliferation of hepatoma cells. The stem-like properties of hepatoma cells were analysed by sphere formation and clone formation assays. The effect of HOXB7 on expression of cancer stem cell markers was evaluated. Transwell and wound-healing assays were performed to estimate the invasion and migration abilities of hepatoma cells. A xenograft tumor model was established in nude mice to assess the role of HOXB7 in tumor growth. Bioluminescence imaging was used to survey the effect of HOXB7 on the metastatic ability of hepatoma cells in vivo.
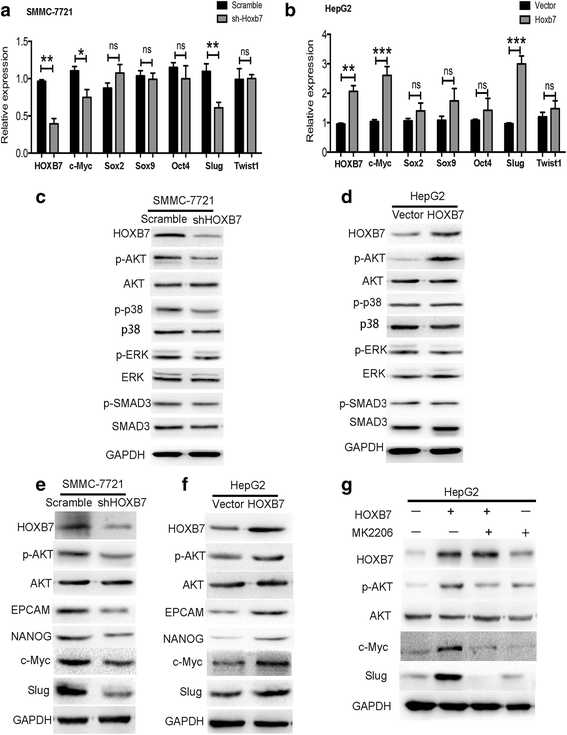
Results: Higher expression of HOXB7 was detected in HCC tissues compared with noncancerous tissues and significantly associated with poor prognosis of HCC. In addition, HOXB7 knockdown suppressed the cell proliferation, clone formation, sphere formation, invasion and migration of hepatoma cells in vitro; conversely, these biological abilities of hepatoma cells were enhanced by HOXB7 overexpression. Moreover, the cancer stem cell markers EPCAM and NANOG were up-regulated by HOXB7. The role of HOXB7 in promoting tumor growth and metastasis was verified in vivo. Further investigation revealed that c-Myc and Slug expression was elevated by HOXB7 and the AKT pathway was activated.
Conclusion: Overexpression of HOXB7 was significantly correlated with poor prognosis of HCC. HOXB7 up-regulated c-Myc and Slug expression via the AKT pathway to promote the acquisition of stem-like properties and facilitate epithelial-mesenchymal transition of hepatoma cells, accelerating the malignant progression of HCC.
Keywords: Epithelial-mesenchymal transition; Hepatocellular carcinoma; Homeobox B7; Slug; Stemness; c-Myc.
Conflict of interest statement
Ethics approval and consent to participate
All patients were informed with consent according to protocols approved by the Institutional Review Board of the Southwest Hospital, Third Military Medical University, and this study complied with by the ethical guidelines of the Helsinki Declaration. Moreover, the animal research was approved by the Animal Research Ethics Committee of Third Military Medical University, and complied with the Guidelines for Animal Experiments of Laboratory Animals.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
HOXB7 promotes tumor progression via bFGF-induced activation of MAPK/ERK pathway and indicated poor prognosis in hepatocellular carcinoma.Oncotarget. 2017 Jul 18;8(29):47121-47135. doi: 10.18632/oncotarget.17004. Oncotarget. 2017. PMID: 28454092 Free PMC article.
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling.Cancer Lett. 2015 Mar 28;358(2):124-135. doi: 10.1016/j.canlet.2014.11.044. Epub 2014 Nov 24. Cancer Lett. 2015. PMID: 25462858
-
MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression.BMC Cancer. 2018 Feb 20;18(1):200. doi: 10.1186/s12885-018-4056-8. BMC Cancer. 2018. PMID: 29463213 Free PMC article. Review.
-
Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications.World J Gastroenterol. 2018 Sep 7;24(33):3695-3708. doi: 10.3748/wjg.v24.i33.3695. World J Gastroenterol. 2018. PMID: 30197476 Free PMC article. Review.
Cited by
-
Circ_0015756 promotes proliferation, invasion and migration by microRNA-7-dependent inhibition of FAK in hepatocellular carcinoma.Cell Cycle. 2019 Nov;18(21):2939-2953. doi: 10.1080/15384101.2019.1664223. Epub 2019 Sep 16. Cell Cycle. 2019. PMID: 31522588 Free PMC article.
-
HOXB7 acts as an oncogenic biomarker in head and neck squamous cell carcinoma.Cancer Cell Int. 2021 Jul 24;21(1):393. doi: 10.1186/s12935-021-02093-6. Cancer Cell Int. 2021. PMID: 34303375 Free PMC article.
-
The role of liver cancer stem cells in hepatocellular carcinoma metastasis.Cancer Biol Ther. 2024 Dec 31;25(1):2321768. doi: 10.1080/15384047.2024.2321768. Epub 2024 Feb 23. Cancer Biol Ther. 2024. PMID: 38393655 Free PMC article. Review.
-
Identification and experimental verification of senescence-related gene signatures and molecular subtypes in idiopathic pulmonary arterial hypertension.Sci Rep. 2024 Sep 27;14(1):22157. doi: 10.1038/s41598-024-72979-8. Sci Rep. 2024. PMID: 39333589 Free PMC article.
-
Homeobox Genes and Hepatocellular Carcinoma.Cancers (Basel). 2019 May 3;11(5):621. doi: 10.3390/cancers11050621. Cancers (Basel). 2019. PMID: 31058850 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

