Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor
- PMID: 28645621
- PMCID: PMC5563499
- DOI: 10.1016/j.neuropharm.2017.06.019
Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor
Abstract
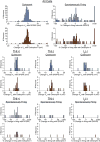
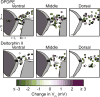
The mu and delta opioid receptors (MOR and DOR) are highly homologous members of the opioid family of GPCRs. There is evidence that MOR and DOR interact, however the extent to which these interactions occur in vivo and affect synaptic function is unknown. There are two stable DOR subtypes: DPDPE sensitive (DOR1) and deltorphin II sensitive (DOR2); both agonists are blocked by DOR selective antagonists. Robust motivational effects are produced by local actions of both MOR and DOR ligands in the ventral tegmental area (VTA). Here we demonstrate that a majority of both dopaminergic and non-dopaminergic VTA neurons express combinations of functional DOR1, DOR2, and/or MOR, and that within a single VTA neuron, DOR1, DOR2, and MOR agonists can differentially couple to downstream signaling pathways. As reported for the MOR agonist DAMGO, DPDPE and deltorphin II produced either a predominant K+ dependent hyperpolarization or a Cav2.1 mediated depolarization in different neurons. In some neurons DPDPE and deltorphin II produced opposite responses. Excitation, inhibition, or no effect by DAMGO did not predict the response to DPDPE or deltorphin II, arguing against a MOR-DOR interaction generating DOR subtypes. However, in a subset of VTA neurons the DOR antagonist TIPP-Ψ augmented DAMGO responses; we also observed DPDPE or deltorphin II responses augmented by the MOR selective antagonist CTAP. These findings directly support the existence of two independent, stable forms of the DOR, and show that MOR and DOR can interact in some neurons to alter downstream signaling.
Keywords: Ca(v)2.1; D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH(2) (CTAP) (PubChem CID: 10418702); Delta opioid receptor; H-Tyr-Tic-psi(CH(2)NH)Phe-Phe-OH (TIPP-psi) (PubChem CID: 5311481); Ventral tegmental area; [D-Ala(2), Glu(4)]deltorphin (deltorphin II) (PubChem CID: 123795); [D-Ala(2), N-Me-Phe(4), Gly-ol(5)]-Enkephalin acetate salt (DAMGO) (PubChem CID: 5462471); [D-Pen(2), D-Pen(5)]enkephalin (DPDPE) (PubChem CID:104787); mu opioid receptor.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

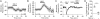
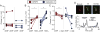

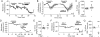

Similar articles
-
Presynaptic modulation of synaptic transmission by opioid receptor in rat subthalamic nucleus in vitro.J Physiol. 2002 May 15;541(Pt 1):219-30. doi: 10.1113/jphysiol.2001.013404. J Physiol. 2002. PMID: 12015431 Free PMC article.
-
Accumbal core: essential link in feed-forward spiraling striato-nigro-striatal in series connected loop.Neuroscience. 2013 Nov 12;252:60-7. doi: 10.1016/j.neuroscience.2013.07.066. Epub 2013 Aug 8. Neuroscience. 2013. PMID: 23933312
-
delta- and mu-opioid receptor mobilization of intracellular calcium in SH-SY5Y human neuroblastoma cells.Br J Pharmacol. 1996 Jan;117(2):333-40. doi: 10.1111/j.1476-5381.1996.tb15195.x. Br J Pharmacol. 1996. PMID: 8789387 Free PMC article.
-
Endogenous opiates and behavior: 2012.Peptides. 2013 Dec;50:55-95. doi: 10.1016/j.peptides.2013.10.001. Epub 2013 Oct 12. Peptides. 2013. PMID: 24126281 Review.
-
Interaction and regulatory functions of μ- and δ-opioid receptors in nociceptive afferent neurons.Neurosci Bull. 2012 Apr;28(2):121-30. doi: 10.1007/s12264-012-1206-x. Neurosci Bull. 2012. PMID: 22466123 Free PMC article. Review.
Cited by
-
Mechanical stimulation of cervical vertebrae modulates the discharge activity of ventral tegmental area neurons and dopamine release in the nucleus accumbens.Brain Stimul. 2020 Mar-Apr;13(2):403-411. doi: 10.1016/j.brs.2019.11.012. Epub 2019 Dec 4. Brain Stimul. 2020. PMID: 31866493 Free PMC article.
-
Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine.Neuropharmacology. 2019 Nov 1;158:107609. doi: 10.1016/j.neuropharm.2019.04.015. Epub 2019 Apr 19. Neuropharmacology. 2019. PMID: 31009632 Free PMC article. Review.
-
Mu and Delta Opioid Receptors Are Coexpressed and Functionally Interact in the Enteric Nervous System of the Mouse Colon.Cell Mol Gastroenterol Hepatol. 2020;9(3):465-483. doi: 10.1016/j.jcmgh.2019.11.006. Epub 2019 Nov 20. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31759144 Free PMC article.
-
Opioid-Induced Signaling and Antinociception Are Modulated by the Recently Deorphanized Receptor, GPR171.J Pharmacol Exp Ther. 2019 Oct;371(1):56-62. doi: 10.1124/jpet.119.259242. Epub 2019 Jul 15. J Pharmacol Exp Ther. 2019. PMID: 31308196 Free PMC article.
-
Characterization of a Multiple-Scan-Rate Voltammetric Waveform for Real-Time Detection of Met-Enkephalin.ACS Chem Neurosci. 2019 Apr 17;10(4):2022-2032. doi: 10.1021/acschemneuro.8b00351. Epub 2019 Jan 12. ACS Chem Neurosci. 2019. PMID: 30571911 Free PMC article.
References
-
- Appelmans N, Carroll JA, Rance MJ, Simon EJ, Traynor JR. Sodium ions increase the binding of the antagonist peptide ICI 174864 to the delta-opiate receptor. Neuropeptides. 1986;7:139–143. - PubMed
-
- Badiani A, Leone P, Noel MB, Stewart J. Ventral tegmental area opioid mechanisms and modulation of ingestive behavior. Brain Res. 1995;670:264–276. - PubMed
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

