Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1β/NF-κB pathway
- PMID: 28643466
- PMCID: PMC5706500
- DOI: 10.1111/jcmm.13227
Anemonin attenuates osteoarthritis progression through inhibiting the activation of IL-1β/NF-κB pathway
Abstract
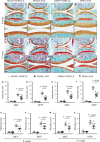
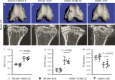
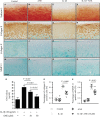
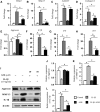
The osteoarthritis (OA) progression is now considered to be related to inflammation. Anemonin (ANE) is a small natural molecule extracted from various kinds of Chinese traditional herbs and has been shown to inhibiting inflammation response. In this study, we examined whether ANE could attenuate the progression of OA via suppression of IL-1β/NF-κB pathway activation. Destabilization of the medial meniscus (DMM) was performed in 10-week-old male C57BL/6J mice. ANE was then intra-articularly injected into joint capsule for 8 and 12 weeks. Human articular chondrocytes and cartilage explants challenged with interleukin-1β (IL-1β) were treated with ANE. We found that ANE delayed articular cartilage degeneration in vitro and in vivo. In particular, proteoglycan loss and chondrocyte hypertrophy were significantly decreased in ANE -treated mice compared with vehicle-treated mice. ANE decreased the expressions of matrix metalloproteinase-13 (MMP13), A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), collagen X (Col X) while increasing Aggrecan level in murine with DMM surgery. ANE treatment also attenuated proteoglycan loss in human cartilage explants treated with IL-1β ex vivo. ANE is a potent protective molecule for OA; it delays OA progression by suppressing ECM loss and chondrocyte hypertrophy partially by suppressing IL-1β/NF-κB pathway activation.
Keywords: NF-κB; anemonin; cartilage; osteoarthritis.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting NF-κB and Wnt/β-catenin signaling in-vitro and in-vivo.Int Immunopharmacol. 2018 Aug;61:222-230. doi: 10.1016/j.intimp.2018.05.026. Epub 2018 Jun 8. Int Immunopharmacol. 2018. PMID: 29890416
-
Lithium protects against cartilage degradation in osteoarthritis.Arthritis Rheumatol. 2014 May;66(5):1228-36. doi: 10.1002/art.38373. Arthritis Rheumatol. 2014. PMID: 24470226
-
Asiatic acid protects articular cartilage through promoting chondrogenesis and inhibiting inflammation and hypertrophy in osteoarthritis.Eur J Pharmacol. 2021 Sep 15;907:174265. doi: 10.1016/j.ejphar.2021.174265. Epub 2021 Jun 24. Eur J Pharmacol. 2021. PMID: 34174266
-
Ghrelin prevents articular cartilage matrix destruction in human chondrocytes.Biomed Pharmacother. 2018 Feb;98:651-655. doi: 10.1016/j.biopha.2017.12.050. Epub 2018 Jan 4. Biomed Pharmacother. 2018. PMID: 29291551 Review.
-
In vivo osteoarthritis target validation utilizing genetically-modified mice.Curr Drug Targets. 2007 Feb;8(2):367-76. doi: 10.2174/138945007779940061. Curr Drug Targets. 2007. PMID: 17305514 Review.
Cited by
-
Anti-Inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes.Biomolecules. 2020 Jun 19;10(6):932. doi: 10.3390/biom10060932. Biomolecules. 2020. PMID: 32575510 Free PMC article.
-
miR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2.Stem Cells Int. 2022 Aug 25;2022:9455152. doi: 10.1155/2022/9455152. eCollection 2022. Stem Cells Int. 2022. PMID: 36061148 Free PMC article.
-
The Therapeutic Effects of Treadmill Exercise on Osteoarthritis in Rats by Inhibiting the HDAC3/NF-KappaB Pathway in vivo and in vitro.Front Physiol. 2019 Aug 20;10:1060. doi: 10.3389/fphys.2019.01060. eCollection 2019. Front Physiol. 2019. PMID: 31481898 Free PMC article.
-
Pseudolaric acid B ameliorates synovial inflammation and vessel formation by stabilizing PPARγ to inhibit NF-κB signalling pathway.J Cell Mol Med. 2021 Jul;25(14):6664-6678. doi: 10.1111/jcmm.16670. Epub 2021 Jun 11. J Cell Mol Med. 2021. PMID: 34117708 Free PMC article.
-
In Vitro Antileishmanial and Antischistosomal Activities of Anemonin Isolated from the Fresh Leaves of Ranunculus multifidus Forsk.Molecules. 2021 Dec 10;26(24):7473. doi: 10.3390/molecules26247473. Molecules. 2021. PMID: 34946555 Free PMC article.
References
-
- Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007; 65: 222–8. - PubMed
-
- Hawker GA, Mian S, Bednis K. Osteoarthritis year 2010 in review: non‐pharmacologic therapy. Osteoarthr Cartil. 2011; 19: 366–74. - PubMed
-
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011; 377: 2115–26. - PubMed
-
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013; 21: 16–21. - PubMed
-
- Pelletier JP, Martel‐Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001; 44: 1237–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

