Prp40 Homolog A Is a Novel Centrin Target
- PMID: 28636910
- PMCID: PMC5479147
- DOI: 10.1016/j.bpj.2017.03.042
Prp40 Homolog A Is a Novel Centrin Target
Abstract
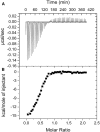
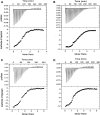
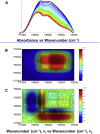
Pre-mRNA processing protein 40 (Prp40) is a nuclear protein that has a role in pre-mRNA splicing. Prp40 possesses two leucine-rich nuclear export signals, but little is known about the function of Prp40 in the export process. Another protein that has a role in protein export is centrin, a member of the EF-hand superfamily of Ca2+-binding proteins. Prp40 was found to be a centrin target by yeast-two-hybrid screening using both Homo sapiens centrin 2 (Hscen2) and Chlamydomonas reinhardtii centrin (Crcen). We identified a centrin-binding site within H. sapiens Prp40 homolog A (HsPrp40A), which contains a hydrophobic triad W1L4L8 that is known to be important in the interaction with centrin. This centrin-binding site is highly conserved within the first nuclear export signal consensus sequence identified in Saccharomyces cerevisiae Prp40. Here, we examine the interaction of HsPrp40A peptide (HsPrp40Ap) with both Hscen2 and Crcen by isothermal titration calorimetry. We employed the thermodynamic parameterization to estimate the polar and apolar surface area of the interface. In addition, we have defined the molecular mechanism of thermally induced unfolding and dissociation of the Crcen-HsPrp40Ap complex using two-dimensional infrared correlation spectroscopy. These complementary techniques showed for the first time, to our knowledge, that HsPrp40Ap interacts with centrin in vitro, supporting a coupled functional role for these proteins in pre-mRNA splicing.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Scherffelia dubia centrin exhibits a specific mechanism for Ca(2+)-controlled target binding.Biochemistry. 2010 May 25;49(20):4383-94. doi: 10.1021/bi901764m. Biochemistry. 2010. PMID: 20408559
-
Structural and thermodynamic studies of two centrin isoforms from Blastocladiella emersonii upon calcium binding.Biochim Biophys Acta. 2013 Dec;1834(12):2823-31. doi: 10.1016/j.bbapap.2013.10.007. Epub 2013 Oct 22. Biochim Biophys Acta. 2013. PMID: 24157662
-
The structure, molecular dynamics, and energetics of centrin-melittin complex.Proteins. 2011 Nov;79(11):3132-43. doi: 10.1002/prot.23142. Epub 2011 Aug 30. Proteins. 2011. PMID: 21989934 Free PMC article.
-
Prp40 and early events in splice site definition.Wiley Interdiscip Rev RNA. 2016 Jan-Feb;7(1):17-32. doi: 10.1002/wrna.1312. Epub 2015 Oct 23. Wiley Interdiscip Rev RNA. 2016. PMID: 26494226 Review.
-
Centrin, centrosomes, and mitotic spindle poles.Curr Opin Cell Biol. 1995 Feb;7(1):39-45. doi: 10.1016/0955-0674(95)80043-3. Curr Opin Cell Biol. 1995. PMID: 7755988 Review.
Cited by
-
Binding by calmodulin is coupled to transient unfolding of the third FF domain of Prp40A.Protein Sci. 2023 Apr;32(4):e4606. doi: 10.1002/pro.4606. Protein Sci. 2023. PMID: 36810829 Free PMC article.
-
Conformational Plasticity of Centrin 1 from Toxoplasma gondii in Binding to the Centrosomal Protein SFI1.Biomolecules. 2022 Aug 13;12(8):1115. doi: 10.3390/biom12081115. Biomolecules. 2022. PMID: 36009009 Free PMC article.
-
Structural Basis for the Functional Diversity of Centrins: A Focus on Calcium Sensing Properties and Target Recognition.Int J Mol Sci. 2021 Nov 10;22(22):12173. doi: 10.3390/ijms222212173. Int J Mol Sci. 2021. PMID: 34830049 Free PMC article. Review.
-
The splicing-factor Prp40 affects dynein-dynactin function in Aspergillus nidulans.Mol Biol Cell. 2020 Jun 1;31(12):1289-1301. doi: 10.1091/mbc.E20-03-0166. Epub 2020 Apr 8. Mol Biol Cell. 2020. PMID: 32267207 Free PMC article.
References
-
- Wahl M.C., Will C.L., Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. - PubMed
-
- Yan C., Hang J., Shi Y. Structure of a yeast spliceosome at 3.6-Å resolution. Science. 2015;349:1182–1191. - PubMed
-
- Abovich N., Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. - PubMed
-
- Berglund J.A., Chua K., Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

