Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors
- PMID: 28634835
- PMCID: PMC5478551
- DOI: 10.1186/s40634-017-0097-1
Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors
Abstract
Background: Evaluation of gene-based approaches to target human bone marrow aspirates in conditions of mechanical stimulation that aim at reproducing the natural joint environment may allow to develop improved treatments for articular cartilage injuries. In the present study, we investigated the potential of rAAV-mediated sox9 gene transfer to enhance the chondrogenic differentiation processes in human bone marrow aspirates under established hydrodynamic conditions compared with the more commonly employed static culture conditions.
Methods: Fresh human bone marrow aspirates were transduced with rAAV-FLAG-hsox9 (40 μl) and maintained for up to 28 days in chondrogenic medium under mechanically-induced conditions in dynamic flow rotating bioreactors that permit tissue growth and matrix deposition relative to static culture conditions. The samples were then processed to examine the potential effects of sox9 overexpression on the cellular activities (matrix synthesis, proliferation) and on the chondrogenic differentiation potency compared with control treatments (absence of rAAV vector; reporter rAAV-lacZ, rAAV-RFP, and rAAV-luc gene transfer).
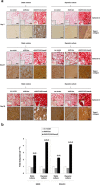
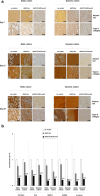
Results: Prolonged, significant sox9 overexpression via rAAV was achieved in the aspirates for at least 28 days when applying the rAAV-FLAG-hsox9 construct, leading to higher, prolonged levels of matrix biosynthesis and to enhanced chondrogenic activities relative to control treatments especially when maintaining the samples under mechanical stimulation. Administration of sox9 however did not impact the indices of proliferation in the aspirates. Remarkably, sox9 gene transfer also durably delayed hypertrophic and osteogenic differentiation in the samples regardless of the conditions of culture applied versus control treatments.
Conclusions: The current observations show the value of genetically modifying human bone marrow aspirates upon mechanical stimulation by rAAV sox9 as a promising strategy for future treatments to improve cartilage repair by implantation in lesions where the tissue is submitted to natural mechanical forces.
Keywords: Bone marrow aspirates; Cartilage repair; Chondrogenic differentiation; Mechanical stimulation; Recombinant adeno-associated virus.
Figures

Similar articles
-
Effects of combined rAAV-mediated TGF-β and sox9 gene transfer and overexpression on the metabolic and chondrogenic activities in human bone marrow aspirates.J Exp Orthop. 2017 Dec;4(1):4. doi: 10.1186/s40634-017-0077-5. Epub 2017 Feb 7. J Exp Orthop. 2017. PMID: 28176272 Free PMC article.
-
Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer.Pharmaceutics. 2020 Mar 21;12(3):280. doi: 10.3390/pharmaceutics12030280. Pharmaceutics. 2020. PMID: 32245159 Free PMC article.
-
rAAV-mediated overexpression of sox9, TGF-β and IGF-I in minipig bone marrow aspirates to enhance the chondrogenic processes for cartilage repair.Gene Ther. 2016 Mar;23(3):247-55. doi: 10.1038/gt.2015.106. Epub 2015 Nov 19. Gene Ther. 2016. PMID: 26583804
-
SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells.Stem Cell Res Ther. 2012;3(3):22. doi: 10.1186/scrt113. Stem Cell Res Ther. 2012. PMID: 22742415 Free PMC article.
-
Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing.Acta Orthop Suppl. 2007 Apr;78(325):1-64. Acta Orthop Suppl. 2007. PMID: 17427340 Review.
Cited by
-
Therapeutic Delivery of rAAV sox9 via Polymeric Micelles Counteracts the Effects of Osteoarthritis-Associated Inflammatory Cytokines in Human Articular Chondrocytes.Nanomaterials (Basel). 2020 Jun 25;10(6):1238. doi: 10.3390/nano10061238. Nanomaterials (Basel). 2020. PMID: 32630578 Free PMC article.
-
Chondrogenic Differentiation of Human Mesenchymal Stem Cells via SOX9 Delivery in Cationic Niosomes.Pharmaceutics. 2022 Oct 28;14(11):2327. doi: 10.3390/pharmaceutics14112327. Pharmaceutics. 2022. PMID: 36365145 Free PMC article.
-
Ultrasonic Enhancement of Chondrogenesis in Mesenchymal Stem Cells by Bolt-Clamped Langevin Transducers.Micromachines (Basel). 2023 Jan 13;14(1):202. doi: 10.3390/mi14010202. Micromachines (Basel). 2023. PMID: 36677263 Free PMC article.
-
Where is human-based cellular pharmaceutical R&D taking us in cartilage regeneration?3 Biotech. 2020 Apr;10(4):161. doi: 10.1007/s13205-020-2134-5. Epub 2020 Mar 6. 3 Biotech. 2020. PMID: 32206495 Free PMC article. Review.
-
Cartilage tissue engineering using decellularized biomatrix hydrogel containing TGF-β-loaded alginate microspheres in mechanically loaded bioreactor.Sci Rep. 2024 May 25;14(1):11991. doi: 10.1038/s41598-024-62474-5. Sci Rep. 2024. PMID: 38796487 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

