Biophysical Mechanisms Mediating Fibrin Fiber Lysis
- PMID: 28630861
- PMCID: PMC5467299
- DOI: 10.1155/2017/2748340
Biophysical Mechanisms Mediating Fibrin Fiber Lysis
Abstract
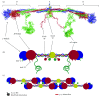
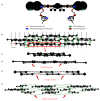
The formation and dissolution of blood clots is both a biochemical and a biomechanical process. While much of the chemistry has been worked out for both processes, the influence of biophysical properties is less well understood. This review considers the impact of several structural and mechanical parameters on lytic rates of fibrin fibers. The influences of fiber and network architecture, fiber strain, FXIIIa cross-linking, and particle transport phenomena will be assessed. The importance of the mechanical aspects of fibrinolysis is emphasized, and future research avenues are discussed.
Figures
Similar articles
-
Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII.J Thromb Haemost. 2016 Jul;14(7):1453-61. doi: 10.1111/jth.13354. Epub 2016 Jun 9. J Thromb Haemost. 2016. PMID: 27148673
-
Mass spectrometry-based molecular mapping of native FXIIIa cross-links in insoluble fibrin clots.J Biol Chem. 2019 May 31;294(22):8773-8778. doi: 10.1074/jbc.AC119.007981. Epub 2019 Apr 26. J Biol Chem. 2019. PMID: 31028172 Free PMC article.
-
Glycaemic control improves fibrin network characteristics in type 2 diabetes - a purified fibrinogen model.Thromb Haemost. 2008 Apr;99(4):691-700. doi: 10.1160/TH07-11-0699. Thromb Haemost. 2008. PMID: 18392327 Free PMC article.
-
Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges.Int J Mol Sci. 2021 Jun 28;22(13):6916. doi: 10.3390/ijms22136916. Int J Mol Sci. 2021. PMID: 34203139 Free PMC article. Review.
-
Cross-linked gamma-chains in fibrin fibrils bridge transversely between strands: no.J Thromb Haemost. 2004 Mar;2(3):394-9. doi: 10.1111/j.1538-7933.2003.00621.x. J Thromb Haemost. 2004. PMID: 15009453 Review. No abstract available.
Cited by
-
Improving the solubility, activity, and stability of reteplase using in silico design of new variants.Res Pharm Sci. 2019 Aug;14(4):359-368. doi: 10.4103/1735-5362.263560. Res Pharm Sci. 2019. PMID: 31516513 Free PMC article.
-
SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19.Biosci Rep. 2021 Aug 27;41(8):BSR20210611. doi: 10.1042/BSR20210611. Biosci Rep. 2021. PMID: 34328172 Free PMC article.
-
Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer.Cancers (Basel). 2021 Mar 22;13(6):1441. doi: 10.3390/cancers13061441. Cancers (Basel). 2021. PMID: 33809973 Free PMC article. Review.
-
Neutrophil extracellular traps and DNases orchestrate formation of peritoneal adhesions.iScience. 2023 Oct 27;26(12):108289. doi: 10.1016/j.isci.2023.108289. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034352 Free PMC article.
-
A critical role for plasminogen in inflammation.J Exp Med. 2020 Apr 6;217(4):e20191865. doi: 10.1084/jem.20191865. J Exp Med. 2020. PMID: 32159743 Free PMC article. Review.
References
-
- Kluft C., Sidelmann J. J., Gram J. B. Assessing safety of thrombolytic therapy. Seminars in Thrombosis and Hemostasis. 2016;43(3):300–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

