SOX2 immunity and tissue resident memory in children and young adults with glioma
- PMID: 28620836
- PMCID: PMC7906294
- DOI: 10.1007/s11060-017-2515-8
SOX2 immunity and tissue resident memory in children and young adults with glioma
Abstract
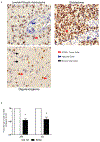
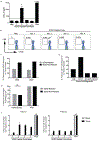
Therapies targeting immune checkpoints are effective in tumors with a high mutation burden that express multiple neo-antigens. However, glial tumors including those seen in children carry fewer mutations and there is an unmet need to identify new antigenic targets of anti-tumor immunity. SOX2 is an embryonal stem cell antigen implicated in the biology of glioma initiating cells. Expression of SOX2 by pediatric glial tumors and the capacity of the immune system in these patients to recognize SOX2 has not been previously studied. We examined the expression of SOX2 on archived paraffin-embedded tissue from pediatric glial tumors. The presence of T-cell immunity to SOX2 was examined in both blood and tumor-infiltrating T-cells in children and young adults with glioma. The nature of tumor-infiltrating immune cells was analyzed with a 37-marker panel using single-cell mass cytometry. SOX2 is expressed by tumor cells but not surrounding normal tissue in pediatric gliomas of all grades. T-cells against this antigen can be detected in blood and tumor tissue in glioma patients. Glial tumors are enriched for CD8/CD4 T-cells with tissue resident memory (TRM; CD45RO+, CD69+, CCR7-) phenotype, which co-express multiple inhibitory checkpoints including PD-1, PD-L1 and TIGIT. Tumors also contain natural killer cells with reduced expression of lytic granzyme. Our data demonstrate immunogenicity of SOX2, which is specifically overexpressed on pediatric glial tumor cells. Harnessing tumor immunity in glioma will likely require the combined targeting of multiple inhibitory checkpoints.
Keywords: Immune checkpoints; Immunotherapy; Pediatric glioma; SOX2.
Conflict of interest statement
Figures


Similar articles
-
Analysis of PD-L1 expression and T cell infiltration in different molecular subgroups of diffuse midline gliomas.Neuropathology. 2019 Dec;39(6):413-424. doi: 10.1111/neup.12594. Epub 2019 Oct 17. Neuropathology. 2019. PMID: 31625205
-
Immune Checkpoint-Associated Locations of Diffuse Gliomas Comparing Pediatric With Adult Patients Based on Voxel-Wise Analysis.Front Immunol. 2021 Mar 17;12:582594. doi: 10.3389/fimmu.2021.582594. eCollection 2021. Front Immunol. 2021. PMID: 33815356 Free PMC article.
-
The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy.J Neuroinflammation. 2018 Oct 17;15(1):290. doi: 10.1186/s12974-018-1330-2. J Neuroinflammation. 2018. PMID: 30333036 Free PMC article.
-
Resident memory T cells, critical components in tumor immunology.J Immunother Cancer. 2018 Sep 4;6(1):87. doi: 10.1186/s40425-018-0399-6. J Immunother Cancer. 2018. PMID: 30180905 Free PMC article. Review.
-
Innate Lymphoid Cells: Expression of PD-1 and Other Checkpoints in Normal and Pathological Conditions.Front Immunol. 2019 Apr 26;10:910. doi: 10.3389/fimmu.2019.00910. eCollection 2019. Front Immunol. 2019. PMID: 31105707 Free PMC article. Review.
Cited by
-
Functional virus-specific memory T cells survey glioblastoma.Cancer Immunol Immunother. 2022 Aug;71(8):1863-1875. doi: 10.1007/s00262-021-03125-w. Epub 2022 Jan 10. Cancer Immunol Immunother. 2022. PMID: 35001153 Free PMC article.
-
SOX2 promotes resistance of melanoma with PD-L1 high expression to T-cell-mediated cytotoxicity that can be reversed by SAHA.J Immunother Cancer. 2020 Nov;8(2):e001037. doi: 10.1136/jitc-2020-001037. J Immunother Cancer. 2020. PMID: 33158915 Free PMC article.
-
Pan-Cancer Analysis Reveals SOX2 as a Promising Prognostic and Immunotherapeutic Biomarker Across Various Cancer Types, Including Pancreatic Cancer.J Cancer. 2024 Jan 1;15(1):251-274. doi: 10.7150/jca.88397. eCollection 2024. J Cancer. 2024. PMID: 38164286 Free PMC article.
-
SOX2 in cancer stemness: tumor malignancy and therapeutic potentials.J Mol Cell Biol. 2020 Feb 20;12(2):85-98. doi: 10.1093/jmcb/mjy080. J Mol Cell Biol. 2020. PMID: 30517668 Free PMC article.
-
Beyond the message: advantages of snapshot proteomics with single-cell mass cytometry in solid tumors.FEBS J. 2019 Apr;286(8):1523-1539. doi: 10.1111/febs.14730. Epub 2019 Jan 7. FEBS J. 2019. PMID: 30549207 Free PMC article. Review.
References
-
- Curtin SCMA, Anderson RN. (20016) Declines in cancer death rates among children and adolescents in the United States, 1999-2014. . NCHS data brief; 257 - PubMed
-
- Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall M, Yasui Y, Nicholson HS, Wolden S, McNeil DE, Mertens AC, Robison LL, Childhood Cancer Survivor S (2003) Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97 (3):663–673. doi:10.1002/cncr.11095 - DOI - PubMed
-
- Jones C, Karajannis MA, Jones DT, Kieran MW, Monje M, Baker SJ, Becher OJ, Cho YJ, Gupta N, Hawkins C, Hargrave D, Haas-Kogan DA, Jabado N, Li XN, Mueller S, Nicolaides T, Packer RJ, Persson AI, Phillips JJ, Simonds EF, Stafford JM, Tang Y, Pfister SM, Weiss WA (2016) Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. doi:10.1093/neuonc/now101 - DOI - PMC - PubMed
-
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS (2016) Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165 (1):35–44. doi:10.1016/j.cell.2016.02.065 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

