Inhibition of the TRPM2 and TRPV1 Channels through Hypericum perforatum in Sciatic Nerve Injury-induced Rats Demonstrates their Key Role in Apoptosis and Mitochondrial Oxidative Stress of Sciatic Nerve and Dorsal Root Ganglion
- PMID: 28620309
- PMCID: PMC5449501
- DOI: 10.3389/fphys.2017.00335
Inhibition of the TRPM2 and TRPV1 Channels through Hypericum perforatum in Sciatic Nerve Injury-induced Rats Demonstrates their Key Role in Apoptosis and Mitochondrial Oxidative Stress of Sciatic Nerve and Dorsal Root Ganglion
Abstract
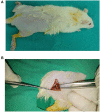
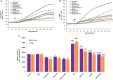
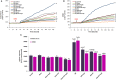
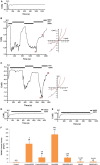
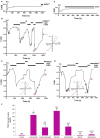
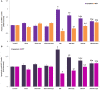
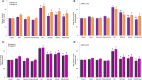
Sciatic nerve injury (SNI) results in neuropathic pain, which is characterized by the excessive Ca2+ entry, reactive oxygen species (ROS) and apoptosis processes although involvement of antioxidant Hypericum perforatum (HP) through TRPM2 and TRPV1 activation has not been clarified on the processes in SNI-induced rat, yet. We investigated the protective property of HP on the processes in the sciatic nerve and dorsal root ganglion neuron (DRGN) of SNI-induced rats. The rats were divided into five groups as control, sham, sham+HP, SNI, and SNI+HP. The HP groups received 30 mg/kg HP for 4 weeks after SNI induction. TRPM2 and TRPV1 channels were activated in the neurons by ADP-ribose or cumene peroxide and capsaicin, respectively. The SNI-induced TRPM2 and TRPV1 currents and intracellular free Ca2+ and ROS concentrations were reduced by HP, N-(p-amylcinnamoyl) anthranilic acid (ACA), and capsazepine (CapZ). SNI-induced increase in apoptosis and mitochondrial depolarization in sciatic nerve and DRGN of SNI group were decreased by HP, ACA, and CapZ treatments. PARP-1, caspase 3 and 9 expressions in the sciatic nerve, DRGN, skin, and musculus piriformis of SNI group were also attenuated by HP treatment. In conclusion, increase of mitochondrial ROS, apoptosis, and Ca2+ entry through inhibition of TRPM2 and TRPV1 in the sciatic nerve and DRGN neurons were decreased by HP treatment. The results may be relevant to the etiology and treatment of SNI by HP.
Keywords: Hypericum perforatum; TRPM2; TRPV1; apoptosis; mitochondrial oxidative stress; sciatic nerve injury.
Figures
Similar articles
-
Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels.Mol Neurobiol. 2016 Aug;53(6):3540-3551. doi: 10.1007/s12035-015-9292-1. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099309
-
Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium.Mol Neurobiol. 2017 Apr;54(3):2345-2360. doi: 10.1007/s12035-016-9727-3. Epub 2016 Mar 9. Mol Neurobiol. 2017. PMID: 26957303
-
Ovariectomy-Induced Mitochondrial Oxidative Stress, Apoptosis, and Calcium Ion Influx Through TRPA1, TRPM2, and TRPV1 Are Prevented by 17β-Estradiol, Tamoxifen, and Raloxifene in the Hippocampus and Dorsal Root Ganglion of Rats.Mol Neurobiol. 2017 Dec;54(10):7620-7638. doi: 10.1007/s12035-016-0232-5. Epub 2016 Nov 10. Mol Neurobiol. 2017. PMID: 27832523
-
TRPM2 Non-Selective Cation Channels in Liver Injury Mediated by Reactive Oxygen Species.Antioxidants (Basel). 2021 Aug 3;10(8):1243. doi: 10.3390/antiox10081243. Antioxidants (Basel). 2021. PMID: 34439491 Free PMC article. Review.
-
Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels.Front Physiol. 2018 Feb 14;9:95. doi: 10.3389/fphys.2018.00095. eCollection 2018. Front Physiol. 2018. PMID: 29491840 Free PMC article. Review.
Cited by
-
Neuroprotective Effect of Nypa fruticans Wurmb by Suppressing TRPV1 Following Sciatic Nerve Crush Injury in a Rat.Nutrients. 2020 Aug 27;12(9):2618. doi: 10.3390/nu12092618. Nutrients. 2020. PMID: 32867278 Free PMC article.
-
Selenium Enhances the Apoptotic Efficacy of Docetaxel Through Activation of TRPM2 Channel in DBTRG Glioblastoma Cells.Neurotox Res. 2019 May;35(4):797-808. doi: 10.1007/s12640-019-0009-5. Epub 2019 Feb 22. Neurotox Res. 2019. PMID: 30796690
-
Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium.Sci Rep. 2017 Dec 13;7(1):17543. doi: 10.1038/s41598-017-17715-1. Sci Rep. 2017. PMID: 29235496 Free PMC article.
-
Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells.Int J Mol Sci. 2020 Jun 11;21(11):4177. doi: 10.3390/ijms21114177. Int J Mol Sci. 2020. PMID: 32545311 Free PMC article. Review.
-
Resveratrol Enhances Apoptotic and Oxidant Effects of Paclitaxel through TRPM2 Channel Activation in DBTRG Glioblastoma Cells.Oxid Med Cell Longev. 2019 Mar 7;2019:4619865. doi: 10.1155/2019/4619865. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30984336 Free PMC article.
References
-
- Carrasco C., Rodriguez A. B., Pariente J. A. (2015). Melatonin as a stabilizer of mitochondrial function: role in diseases and aging. Turk. J. Biol. 39, 822–831. 10.3906/biy-1504-26 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

