Pancreatic adenocarcinoma response to chemotherapy enhanced with non-invasive radio frequency evaluated via an integrated experimental/computational approach
- PMID: 28611425
- PMCID: PMC5469743
- DOI: 10.1038/s41598-017-03040-0
Pancreatic adenocarcinoma response to chemotherapy enhanced with non-invasive radio frequency evaluated via an integrated experimental/computational approach
Abstract
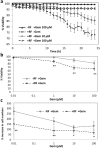
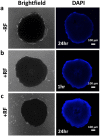
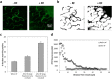
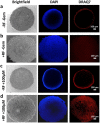
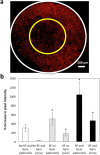
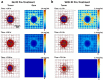
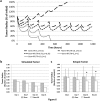
Although chemotherapy combined with radiofrequency exposure has shown promise in cancer treatment by coupling drug cytotoxicity with thermal ablation or thermally-induced cytotoxicity, limited access of the drug to tumor loci in hypo-vascularized lesions has hampered clinical application. We recently showed that high-intensity short-wave capacitively coupled radiofrequency (RF) electric-fields may reach inaccessible targets in vivo. This non-invasive RF combined with gemcitabine (Gem) chemotherapy enhanced drug uptake and effect in pancreatic adenocarcinoma (PDAC), notorious for having poor response and limited therapeutic options, but without inducing thermal injury. We hypothesize that the enhanced cytotoxicity derives from RF-facilitated drug transport in the tumor microenvironment. We propose an integrated experimental/computational approach to evaluate chemotherapeutic response combined with RF-induced phenotypic changes in tissue with impaired transport. Results show that RF facilitates diffusive transport in 3D cell cultures representing hypo-vascularized lesions, enhancing drug uptake and effect. Computational modeling evaluates drug vascular extravasation and diffusive transport as key RF-modulated parameters, with transport being dominant. Assessment of hypothetical schedules following current clinical protocol for Stage-IV PDAC suggests that unresponsive lesions may be growth-restrained when exposed to Gem plus RF. Comparison of these projections to experiments in vivo indicates that synergy may result from RF-induced cell phenotypic changes enhancing drug transport and cytotoxicity, thus providing a potential baseline for clinically-focused evaluation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Autophagy and enhanced chemosensitivity in experimental pancreatic cancers induced by noninvasive radiofrequency field treatment.Cancer. 2014 Feb 15;120(4):480-91. doi: 10.1002/cncr.28453. Epub 2013 Oct 25. Cancer. 2014. PMID: 24496866 Free PMC article.
-
Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma.Cancer Lett. 2017 Sep 10;403:296-304. doi: 10.1016/j.canlet.2017.06.026. Epub 2017 Jul 4. Cancer Lett. 2017. PMID: 28687352 Free PMC article.
-
MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma.Sci Rep. 2017 Feb 15;7:42339. doi: 10.1038/srep42339. Sci Rep. 2017. PMID: 28198398 Free PMC article.
-
Updates on treatment of gemcitabine-refractory pancreatic adenocarcinoma. Highlights from the "2011 ASCO Annual Meeting". Chicago, IL, USA; June 3-7, 2011.JOP. 2011 Jul 8;12(4):351-4. JOP. 2011. PMID: 21737894 Review.
-
Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going.Exp Mol Med. 2017 Dec 1;49(12):e406. doi: 10.1038/emm.2017.255. Exp Mol Med. 2017. PMID: 29611542 Free PMC article. Review.
Cited by
-
Peptide Mediated In Vivo Tumor Targeting of Nanoparticles through Optimization in Single and Multilayer In Vitro Cell Models.Cancers (Basel). 2018 Mar 20;10(3):84. doi: 10.3390/cancers10030084. Cancers (Basel). 2018. PMID: 29558451 Free PMC article.
-
EUS-guided ablation with the HybridTherm Probe as second-line treatment in patients with locally advanced pancreatic ductal adenocarcinoma: A case-control study.Endosc Ultrasound. 2022 Sep-Oct;11(5):383-392. doi: 10.4103/EUS-D-21-00200. Endosc Ultrasound. 2022. PMID: 36255026 Free PMC article.
-
Locoregional Therapies and Remodeling of Tumor Microenvironment in Pancreatic Cancer.Int J Mol Sci. 2023 Aug 11;24(16):12681. doi: 10.3390/ijms241612681. Int J Mol Sci. 2023. PMID: 37628865 Free PMC article. Review.
-
Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer.Endosc Int Open. 2020 Oct;8(10):E1511-E1519. doi: 10.1055/a-1221-9879. Epub 2020 Oct 7. Endosc Int Open. 2020. PMID: 33043122 Free PMC article.
-
Modeling of Combination Chemotherapy and Immunotherapy for Lung Cancer.Annu Int Conf IEEE Eng Med Biol Soc. 2019 Jul;2019:273-276. doi: 10.1109/EMBC.2019.8857566. Annu Int Conf IEEE Eng Med Biol Soc. 2019. PMID: 31945894 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

