Evaluation of focused multipolar stimulation for cochlear implants: a preclinical safety study
- PMID: 28607224
- PMCID: PMC5681383
- DOI: 10.1088/1741-2552/aa7586
Evaluation of focused multipolar stimulation for cochlear implants: a preclinical safety study
Abstract
Objective: Cochlear implants (CIs) have a limited number of independent stimulation channels due to the highly conductive nature of the fluid-filled cochlea. Attempts to develop highly focused stimulation to improve speech perception in CI users includes the use of simultaneous stimulation via multiple current sources. Focused multipolar (FMP) stimulation is an example of this approach and has been shown to reduce interaction between stimulating channels. However, compared with conventional biphasic current pulses generated from a single current source, FMP is a complex stimulus that includes extended periods of stimulation before charge recovery is achieved, raising questions on whether chronic stimulation with this strategy is safe. The present study evaluated the long-term safety of intracochlear stimulation using FMP in a preclinical animal model of profound deafness.
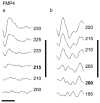
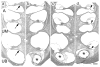
Approach: Six cats were bilaterally implanted with scala tympani electrode arrays two months after deafening, and received continuous unilateral FMP stimulation at levels that evoked a behavioural response for periods of up to 182 d. Electrode impedance, electrically-evoked compound action potentials (ECAPs) and auditory brainstem responses (EABRs) were monitored periodically over the course of the stimulation program from both the stimulated and contralateral control cochleae. On completion of the stimulation program cochleae were examined histologically and the electrode arrays were evaluated for evidence of platinum (Pt) corrosion.
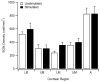
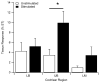
Main results: There was no significant difference in electrode impedance between control and chronically stimulated electrodes following long-term FMP stimulation. Moreover, there was no significant difference between ECAP and EABR thresholds evoked from control or stimulated cochleae at either the onset of stimulation or at completion of the stimulation program. Chronic FMP stimulation had no effect on spiral ganglion neuron (SGN) survival when compared with unstimulated control cochleae. Long-term implantation typically evoked a mild foreign body reaction proximal to the electrode array; however stimulated cochleae exhibited a small but statistically significant increase in the tissue response. Finally, there was no evidence of Pt corrosion following long-term FMP stimulation; stimulated electrodes exhibited the same surface features as the unstimulated control electrodes.
Significance: Chronic intracochlear FMP stimulation at levels used in the present study did not adversely affect electrically-evoked neural thresholds or SGN survival but evoked a small, benign increase in inflammatory response compared to control ears. Moreover chronic FMP stimulation does not affect the surface of Pt electrodes at suprathreshold stimulus levels. These findings support the safe clinical application of an FMP stimulation strategy.
Figures
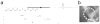


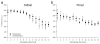



Similar articles
-
Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study.Hear Res. 1997 Mar;105(1-2):1-29. doi: 10.1016/s0378-5955(96)00193-1. Hear Res. 1997. PMID: 9083801
-
Chronic intracochlear electrical stimulation at high charge densities results in platinum dissolution but not neural loss or functional changes in vivo.J Neural Eng. 2019 Apr;16(2):026009. doi: 10.1088/1741-2552/aaf66b. Epub 2018 Dec 5. J Neural Eng. 2019. PMID: 30523828 Free PMC article.
-
Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons?Hear Res. 2007 Mar;225(1-2):60-70. doi: 10.1016/j.heares.2006.12.004. Epub 2006 Dec 15. Hear Res. 2007. PMID: 17258411 Free PMC article.
-
The Panoramic ECAP Method: Estimating Patient-Specific Patterns of Current Spread and Neural Health in Cochlear Implant Users.J Assoc Res Otolaryngol. 2021 Oct;22(5):567-589. doi: 10.1007/s10162-021-00795-2. Epub 2021 Apr 23. J Assoc Res Otolaryngol. 2021. PMID: 33891218 Free PMC article. Review.
-
The Role of Auditory Evoked Potentials in the Context of Cochlear Implant Provision.Otol Neurotol. 2017 Dec;38(10):e522-e530. doi: 10.1097/MAO.0000000000001480. Otol Neurotol. 2017. PMID: 29135872 Review.
Cited by
-
Chronic intracochlear electrical stimulation at high charge densities: reducing platinum dissolution.J Neural Eng. 2020 Oct 8;17(5):056009. doi: 10.1088/1741-2552/abb7a6. J Neural Eng. 2020. PMID: 32916669 Free PMC article.
-
Intracochlear fibrosis and the foreign body response to cochlear implant biomaterials.Laryngoscope Investig Otolaryngol. 2019 Nov 13;4(6):678-683. doi: 10.1002/lio2.329. eCollection 2019 Dec. Laryngoscope Investig Otolaryngol. 2019. PMID: 31890888 Free PMC article. Review.
-
Engineered Graphene Material Improves the Performance of Intraneural Peripheral Nerve Electrodes.Adv Sci (Weinh). 2024 Aug;11(29):e2308689. doi: 10.1002/advs.202308689. Epub 2024 Jun 11. Adv Sci (Weinh). 2024. PMID: 38863325 Free PMC article.
-
Platinum dissolution and tissue response following long-term electrical stimulation at high charge densities.J Neural Eng. 2021 Mar 17;18(3):10.1088/1741-2552/abe5ba. doi: 10.1088/1741-2552/abe5ba. J Neural Eng. 2021. PMID: 33578409 Free PMC article.
-
The development of neural stimulators: a review of preclinical safety and efficacy studies.J Neural Eng. 2018 Aug;15(4):041004. doi: 10.1088/1741-2552/aac43c. Epub 2018 May 14. J Neural Eng. 2018. PMID: 29756600 Free PMC article.
References
-
- AGNEW WF, MCCREERY DB, YUEN TG, BULLARA LA. MK-801 protects against neuronal injury induced by electrical stimulation. Neuroscience. 1993;52:45–53. - PubMed
-
- BIERER JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. The Journal of the Acoustical Society of America. 2007;121:1642–53. - PubMed
-
- BLACK RC, CLARK GM, PATRICK JF. Current distribution measurements within the human cochlea. IEEE transactions on bio-medical engineering. 1981;28:721–5. - PubMed
-
- BOEX C, DE BALTHASAR C, KOS MI, PELIZZONE M. Electrical field interactions in different cochlear implant systems. The Journal of the Acoustical Society of America. 2003;114:2049–57. - PubMed
-
- CEN. EN4550502-2-3. European Committee for Standardization; 2010. Particular requirements for cochlear and auditory brainstem implant systems.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
