Ferrous iron efflux systems in bacteria
- PMID: 28604884
- PMCID: PMC5675029
- DOI: 10.1039/c7mt00112f
Ferrous iron efflux systems in bacteria
Abstract
Bacteria require iron for growth, with only a few reported exceptions. In many environments, iron is a limiting nutrient for growth and high affinity uptake systems play a central role in iron homeostasis. However, iron can also be detrimental to cells when it is present in excess, particularly under aerobic conditions where its participation in Fenton chemistry generates highly reactive hydroxyl radicals. Recent results have revealed a critical role for iron efflux transporters in protecting bacteria from iron intoxication. Systems that efflux iron are widely distributed amongst bacteria and fall into several categories: P1B-type ATPases, cation diffusion facilitator (CDF) proteins, major facilitator superfamily (MFS) proteins, and membrane bound ferritin-like proteins. Here, we review the emerging role of iron export in both iron homeostasis and as part of the adaptive response to oxidative stress.
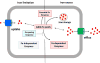
Figures

Similar articles
-
Manganese Efflux Achieved by MetA and MetB Affects Oxidative Stress Resistance and Iron Homeostasis in Riemerella anatipestifer.Appl Environ Microbiol. 2023 Mar 29;89(3):e0183522. doi: 10.1128/aem.01835-22. Epub 2023 Feb 23. Appl Environ Microbiol. 2023. PMID: 36815770 Free PMC article.
-
Ins and Outs: Recent Advancements in Membrane Protein-Mediated Prokaryotic Ferrous Iron Transport.Biochemistry. 2021 Nov 9;60(44):3277-3291. doi: 10.1021/acs.biochem.1c00586. Epub 2021 Oct 20. Biochemistry. 2021. PMID: 34670078 Free PMC article. Review.
-
Sll1263, a unique cation diffusion facilitator protein that promotes iron uptake in the cyanobacterium Synechocystis sp. Strain PCC 6803.Plant Cell Physiol. 2012 Aug;53(8):1404-17. doi: 10.1093/pcp/pcs086. Epub 2012 Jun 7. Plant Cell Physiol. 2012. PMID: 22685083
-
Iron homeostasis in Bacillus subtilis relies on three differentially expressed efflux systems.Microbiology (Reading). 2023 Jan;169(1):001289. doi: 10.1099/mic.0.001289. Microbiology (Reading). 2023. PMID: 36748638 Free PMC article.
-
Bacterial ferrous iron transport: the Feo system.FEMS Microbiol Rev. 2016 Mar;40(2):273-98. doi: 10.1093/femsre/fuv049. Epub 2015 Dec 17. FEMS Microbiol Rev. 2016. PMID: 26684538 Review.
Cited by
-
The Bacillus subtilis yqgC-sodA operon protects magnesium-dependent enzymes by supporting manganese efflux.J Bacteriol. 2024 Jun 20;206(6):e0005224. doi: 10.1128/jb.00052-24. Epub 2024 May 31. J Bacteriol. 2024. PMID: 38819154 Free PMC article.
-
The Bacillus subtilis yqgC-sodA operon protects magnesium-dependent enzymes by supporting manganese efflux.bioRxiv [Preprint]. 2024 Feb 15:2024.02.14.580342. doi: 10.1101/2024.02.14.580342. bioRxiv. 2024. Update in: J Bacteriol. 2024 Jun 20;206(6):e0005224. doi: 10.1128/jb.00052-24. PMID: 38405924 Free PMC article. Updated. Preprint.
-
Faecalibacterium duncaniae A2-165 regulates the expression of butyrate synthesis, ferrous iron uptake, and stress-response genes based on acetate consumption.Sci Rep. 2024 Jan 10;14(1):987. doi: 10.1038/s41598-023-51059-3. Sci Rep. 2024. PMID: 38200051 Free PMC article.
-
The divalent metal ion exporter IhpABC is required to maintain iron homeostasis under low to moderate environmental iron conditions in the bacterium Bradyrhizobium japonicum.Mol Microbiol. 2024 Jan;121(1):85-97. doi: 10.1111/mmi.15198. Epub 2023 Dec 1. Mol Microbiol. 2024. PMID: 38038163
-
Pathogenic bacteria build an iron-storage system for growth and survival in their hosts.Nature. 2023 Nov 15. doi: 10.1038/d41586-023-03367-x. Online ahead of print. Nature. 2023. PMID: 37968459 No abstract available.
References
-
- Imlay JA. Pathways of oxidative damage. Annual review of Microbiology. 2003;57:395–418. - PubMed
-
- Barwinska-Sendra A, Waldron KJ. Advances in Microbial Physiology. Academic Press; DOI: http://doi.org/10.1016/bs.ampbs.2017.01.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

