Phase I study of oral ridaforolimus in combination with paclitaxel and carboplatin in patients with solid tumor cancers
- PMID: 28595616
- PMCID: PMC5465458
- DOI: 10.1186/s12885-017-3394-2
Phase I study of oral ridaforolimus in combination with paclitaxel and carboplatin in patients with solid tumor cancers
Abstract
Background: Ridaforolimus is a mammalian target of rapamycin inhibitor that has activity in solid tumors. Paclitaxel and carboplatin have broad antineoplastic activity in many cancers. This phase I trial was conducted to determine the safety profile, maximal tolerated dose, and recommended phase II dose and schedule of oral ridaforolimus combined with paclitaxel and carboplatin in patients with solid tumor cancers.
Methods: Eligible patients with advanced solid tumor cancers received oral 10 to 30 mg ridaforolimus daily for 5 consecutive days per week combined with intravenous paclitaxel (175 mg/m2) and carboplatin (area under the curve [AUC] 5-6 mg/mL/min) in 3-week cycles. A standard 3 + 3 design was used to escalate doses, with predefined changes to an alternate dosing schedule and/or changes in carboplatin AUC doses based on dose-limiting toxicity (DLT). Secondary information was collected regarding response and time to progression. Patients were continued on treatment if therapy was tolerated and if stable disease or better was demonstrated.
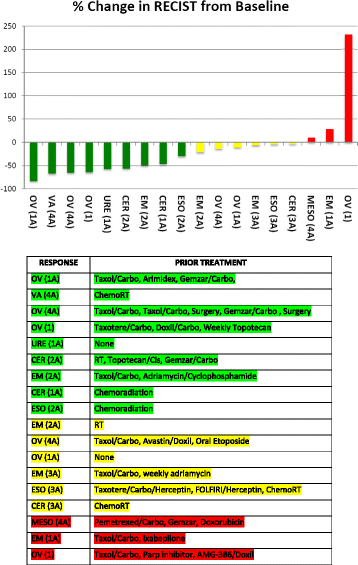
Results: Thirty-one patients were consented, 28 patients were screened, and 24 patients met eligibility requirements and received treatment. Two patients were replaced for events unrelated to drug-related toxicity, resulting in 22 DLT-evaluable patients. Two grade 4 DLTs due to neutropenia were observed at dose level 1. The next cohort was changed to a predefined alternate dosing schedule (days 1-5 and 8-12). DLTs were neutropenia, sepsis, mucositis, and thrombocytopenia. The most common adverse events were neutropenia, anemia, thrombocytopenia, fatigue, alopecia, nausea, pain, and leukopenia. Twenty-four patients received a median of 4 cycles (range, 1-12). Evaluable patients for response (n = 18) demonstrated a median tumor measurement decrease of 25%. The best response in these 18 patients included 9 patients with partial response (50%), 6 with stable disease (33%), and 3 with progressive disease (17%). Thirteen of these patients received treatment for 4 or more cycles.
Conclusions: Treatment with ridaforolimus combined with paclitaxel and carboplatin had no unanticipated toxicities and showed antineoplastic activity. The recommended phase II dose and schedule is ridaforolimus 30 mg (days 1-5 and 8-12) plus day 1 paclitaxel (175 mg/m2) and carboplatin (AUC 5 mg/mL/min) on a 21-day cycle.
Trial registration: ClinicalTrials.gov Identifier: NCT01256268 (trial registration date: December 1, 2010).
Keywords: Oral ridaforolimus; Paclitaxel and carboplatin combination; Phase 1 trial; Solid tumors.
Figures
Similar articles
-
Feasibility of adding everolimus to carboplatin and paclitaxel, with or without bevacizumab, for treatment-naive, advanced non-small cell lung cancer.Invest New Drugs. 2014 Feb;32(1):123-34. doi: 10.1007/s10637-013-9958-3. Epub 2013 Apr 12. Invest New Drugs. 2014. PMID: 23579358
-
Phase I dose-finding and pharmacokinetic study of paclitaxel and carboplatin with oral valspodar in patients with advanced solid tumors.J Clin Oncol. 2000 Nov 1;18(21):3677-89. doi: 10.1200/JCO.2000.18.21.3677. J Clin Oncol. 2000. PMID: 11054441 Clinical Trial.
-
Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study.J Clin Oncol. 2009 Sep 10;27(26):4413-21. doi: 10.1200/JCO.2008.21.7422. Epub 2009 Aug 3. J Clin Oncol. 2009. PMID: 19652058 Clinical Trial.
-
Phase II study of carboplatin-paclitaxel combination chemotherapy in elderly patients with advanced non-small cell lung cancer.Jpn J Clin Oncol. 2005 Apr;35(4):188-94. doi: 10.1093/jjco/hyi059. Jpn J Clin Oncol. 2005. PMID: 15845567 Review.
-
Utility of individualized carboplatin dosing alone and in combination regimens.Semin Oncol. 1992 Feb;19(1 Suppl 2):132-8. Semin Oncol. 1992. PMID: 1411624 Review.
Cited by
-
Targeted therapies in gynecological cancers: a comprehensive review of clinical evidence.Signal Transduct Target Ther. 2020 Jul 29;5(1):137. doi: 10.1038/s41392-020-0199-6. Signal Transduct Target Ther. 2020. PMID: 32728057 Free PMC article. Review.
-
Drug repositioning via host-pathogen protein-protein interactions for the treatment of cervical cancer.Front Oncol. 2023 Jan 25;13:1096081. doi: 10.3389/fonc.2023.1096081. eCollection 2023. Front Oncol. 2023. PMID: 36761959 Free PMC article.
-
Clinical research progress of ridaforolimus (AP23573, MK8668) over the past decade: a systemic review.Front Pharmacol. 2024 Mar 22;15:1173240. doi: 10.3389/fphar.2024.1173240. eCollection 2024. Front Pharmacol. 2024. PMID: 38584599 Free PMC article. Review.
-
Targeting mTOR and Metabolism in Cancer: Lessons and Innovations.Cells. 2019 Dec 6;8(12):1584. doi: 10.3390/cells8121584. Cells. 2019. PMID: 31817676 Free PMC article. Review.
-
New therapies for advanced, recurrent, and metastatic endometrial cancers.Gynecol Oncol Res Pract. 2017 Dec 2;4:19. doi: 10.1186/s40661-017-0056-7. eCollection 2017. Gynecol Oncol Res Pract. 2017. PMID: 29214032 Free PMC article. Review.
References
-
- Rivera VM, Squillace RM, Miller D, Berk L, Wardwell SD, Ning Y, et al. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther. 2011;10(6):1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

