Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development
- PMID: 28592260
- PMCID: PMC5463420
- DOI: 10.1186/s12943-017-0670-3
Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development
Abstract
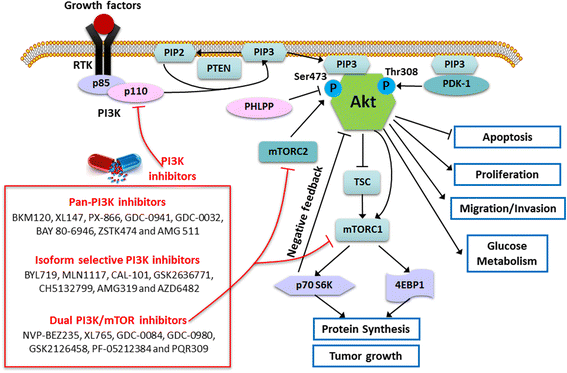
Glioblastoma multiforme (GBM) is the most common and aggressive malignant primary tumor in the central nervous system. One of the most widely used chemotherapeutic drugs for GBM is temozolomide, which is a DNA-alkylating agent and its efficacy is dependent on MGMT methylation status. Little progress in improving the prognosis of GBM patients has been made in the past ten years, urging the development of more effective molecular targeted therapies. Hyper-activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is frequently found in a variety of cancers including GBM, and it plays a central role in the regulation of tumor cell survival, growth, motility, angiogenesis and metabolism. Numerous PI3K inhibitors including pan-PI3K, isoform-selective and dual PI3K/mammalian target of rapamycin (mTOR) inhibitors have exhibited favorable preclinical results and entered clinical trials in a range of hematologic malignancies and solid tumors. Furthermore, combination of inhibitors targeting PI3K and other related pathways may exert synergism on suppressing tumor growth and improving patients' prognosis. Currently, only a handful of PI3K inhibitors are in phase I/II clinical trials for GBM treatment. In this review, we focus on the importance of PI3K/Akt pathway in GBM, and summarize the current development of PI3K inhibitors alone or in combination with other inhibitors for GBM treatment from preclinical to clinical studies.
Keywords: GBM; Glioblastoma; PI3K; mTOR.
Figures
Similar articles
-
PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma.Oncotarget. 2016 May 31;7(22):33440-50. doi: 10.18632/oncotarget.7961. Oncotarget. 2016. PMID: 26967052 Free PMC article. Review.
-
Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: novel therapeutic agents and advances in understanding.Tumour Biol. 2013 Aug;34(4):1991-2002. doi: 10.1007/s13277-013-0800-5. Epub 2013 Apr 30. Tumour Biol. 2013. PMID: 23625692 Review.
-
Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies.Paediatr Drugs. 2012 Oct 1;14(5):299-316. doi: 10.2165/11594740-000000000-00000. Paediatr Drugs. 2012. PMID: 22845486 Free PMC article. Review.
-
MR Studies of Glioblastoma Models Treated with Dual PI3K/mTOR Inhibitor and Temozolomide:Metabolic Changes Are Associated with Enhanced Survival.Mol Cancer Ther. 2016 May;15(5):1113-22. doi: 10.1158/1535-7163.MCT-15-0769. Epub 2016 Feb 16. Mol Cancer Ther. 2016. PMID: 26883274 Free PMC article.
-
A Potential Role for the Inhibition of PI3K Signaling in Glioblastoma Therapy.PLoS One. 2015 Jun 29;10(6):e0131670. doi: 10.1371/journal.pone.0131670. eCollection 2015. PLoS One. 2015. PMID: 26121251 Free PMC article.
Cited by
-
Mass spectrometry imaging discriminates glioblastoma tumor cell subpopulations and different microvascular formations based on their lipid profiles.Sci Rep. 2022 Oct 12;12(1):17069. doi: 10.1038/s41598-022-22093-4. Sci Rep. 2022. PMID: 36224354 Free PMC article.
-
Understanding Glioblastoma Signaling, Heterogeneity, Invasiveness, and Drug Delivery Barriers.Int J Mol Sci. 2023 Sep 19;24(18):14256. doi: 10.3390/ijms241814256. Int J Mol Sci. 2023. PMID: 37762559 Free PMC article. Review.
-
Glioblastoma: Current Status, Emerging Targets, and Recent Advances.J Med Chem. 2022 Jul 14;65(13):8596-8685. doi: 10.1021/acs.jmedchem.1c01946. Epub 2022 Jul 5. J Med Chem. 2022. PMID: 35786935 Free PMC article. Review.
-
Repression of PCGF1 Decreases the Proliferation of Glioblastoma Cells in Association with Inactivation of c-Myc Signaling Pathway.Onco Targets Ther. 2020 Jan 9;13:253-261. doi: 10.2147/OTT.S234517. eCollection 2020. Onco Targets Ther. 2020. PMID: 32021272 Free PMC article.
-
Upregulation of contactin-1 expression promotes prostate cancer progression.Oncol Lett. 2020 Feb;19(2):1611-1618. doi: 10.3892/ol.2019.11244. Epub 2019 Dec 23. Oncol Lett. 2020. PMID: 32002038 Free PMC article.
References
-
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–1387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

