Sox5 regulates beta-cell phenotype and is reduced in type 2 diabetes
- PMID: 28585545
- PMCID: PMC5467166
- DOI: 10.1038/ncomms15652
Sox5 regulates beta-cell phenotype and is reduced in type 2 diabetes
Abstract
Type 2 diabetes (T2D) is characterized by insulin resistance and impaired insulin secretion, but the mechanisms underlying insulin secretion failure are not completely understood. Here, we show that a set of co-expressed genes, which is enriched for genes with islet-selective open chromatin, is associated with T2D. These genes are perturbed in T2D and have a similar expression pattern to that of dedifferentiated islets. We identify Sox5 as a regulator of the module. Sox5 knockdown induces gene expression changes similar to those observed in T2D and diabetic animals and has profound effects on insulin secretion, including reduced depolarization-evoked Ca2+-influx and β-cell exocytosis. SOX5 overexpression reverses the expression perturbations observed in a mouse model of T2D, increases the expression of key β-cell genes and improves glucose-stimulated insulin secretion in human islets from donors with T2D. We suggest that human islets in T2D display changes reminiscent of dedifferentiation and highlight SOX5 as a regulator of β-cell phenotype and function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
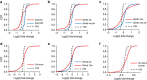
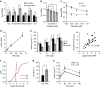
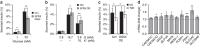
Comment in
-
Diabetes: Why β cells fail in T2DM.Nat Rev Endocrinol. 2017 Aug;13(8):440. doi: 10.1038/nrendo.2017.82. Epub 2017 Jun 23. Nat Rev Endocrinol. 2017. PMID: 28643804 No abstract available.
Similar articles
-
CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion.Diabetologia. 2016 Sep;59(9):1928-37. doi: 10.1007/s00125-016-4020-6. Epub 2016 Jun 23. Diabetologia. 2016. PMID: 27338624
-
A Glycine-Insulin Autocrine Feedback Loop Enhances Insulin Secretion From Human β-Cells and Is Impaired in Type 2 Diabetes.Diabetes. 2016 Aug;65(8):2311-21. doi: 10.2337/db15-1272. Epub 2016 May 3. Diabetes. 2016. PMID: 27207556
-
Elovl6 Deficiency Improves Glycemic Control in Diabetic db/db Mice by Expanding β-Cell Mass and Increasing Insulin Secretory Capacity.Diabetes. 2017 Jul;66(7):1833-1846. doi: 10.2337/db16-1277. Epub 2017 May 1. Diabetes. 2017. PMID: 28461456
-
MicroRNAs in islet hormone secretion.Diabetes Obes Metab. 2018 Sep;20 Suppl 2:11-19. doi: 10.1111/dom.13382. Diabetes Obes Metab. 2018. PMID: 30230181 Review.
-
β-Cell Ca(2+) dynamics and function are compromised in aging.Adv Biol Regul. 2015 Jan;57:112-9. doi: 10.1016/j.jbior.2014.09.005. Epub 2014 Sep 20. Adv Biol Regul. 2015. PMID: 25282681 Review.
Cited by
-
A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides.Food Chem X. 2022 Feb 2;13:100222. doi: 10.1016/j.fochx.2022.100222. eCollection 2022 Mar 30. Food Chem X. 2022. PMID: 35498998 Free PMC article.
-
Genotype-Based Recall Studies in Complex Cardiometabolic Traits.Circ Genom Precis Med. 2018 Aug;11(8):e001947. doi: 10.1161/CIRCGEN.118.001947. Circ Genom Precis Med. 2018. PMID: 30354344 Free PMC article. Review.
-
Therapeutic Effect of P-Cymene on Lipid Profile, Liver Enzyme, and Akt/Mtor Pathway in Streptozotocin-Induced Diabetes Mellitus in Wistar Rats.J Obes. 2022 Apr 26;2022:1015669. doi: 10.1155/2022/1015669. eCollection 2022. J Obes. 2022. PMID: 35528246 Free PMC article.
-
Obesity-induced reduced expression of the lncRNA ROIT impairs insulin transcription by downregulation of Nkx6.1 methylation.Diabetologia. 2020 Apr;63(4):811-824. doi: 10.1007/s00125-020-05090-y. Epub 2020 Feb 1. Diabetologia. 2020. PMID: 32008054
-
Immune cell-derived extracellular vesicular microRNAs induce pancreatic beta cell apoptosis.Heliyon. 2022 Dec 2;8(12):e11995. doi: 10.1016/j.heliyon.2022.e11995. eCollection 2022 Dec. Heliyon. 2022. PMID: 36561684 Free PMC article.
References
-
- DeFronzo R. A. Pathogenesis of type 2 (non-insulin dependent) diabetes mellitus: a balanced overview. Diabetologia 35, 389–397 (1992). - PubMed
-
- Butler A. E. et al.. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003). - PubMed
-
- Rahier J., Guiot Y., Goebbels R. M., Sempoux C. & Henquin J. C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10, 32–42 (2008). - PubMed
-
- Del Guerra S. et al.. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54, 727–735 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

