The disulfide isomerase ERp72 supports arterial thrombosis in mice
- PMID: 28576878
- PMCID: PMC5553574
- DOI: 10.1182/blood-2016-12-755587
The disulfide isomerase ERp72 supports arterial thrombosis in mice
Abstract
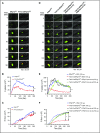
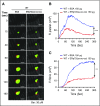
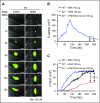
Several CGHC motif-containing disulfide isomerases support thrombosis. We here report that endoplasmic reticulum protein 72 (ERp72), with 3 CGHC redox-active sites (ao, a, and a'), supports thrombosis. We generated a new conditional knockout mouse model and found that Tie2-Cre/ERp72fl/fl mice with blood and endothelial cells lacking ERp72 had prolonged tail bleeding times and decreased platelet accumulation in laser-induced cremaster arteriole injury and FeCl3-induced mesenteric arterial injury. Fibrin deposition was decreased in the laser injury model. Both platelet and fibrin accumulation defects were fully rescued by infusion of recombinant ERp72 containing functional a and a' CGHC motifs (ERp72(oo-ss-ss)). Infusion of ERp72 containing inactivated a and a' CGHC motifs (ERp72(ss-oo-oo)) inhibited platelet accumulation and fibrin deposition in wild-type mice. Infusion of ERp72(oo-ss-ss) into β3-null mice increased fibrin deposition in the absence of platelets. ERp72-null platelets had defective aggregation, JON/A binding, P-selectin expression, and adenosine triphosphate (ATP) secretion. The aggregation and ATP secretion defects were fully rescued by ERp72(oo-ss-ss) but partially rescued by ERp72(ss-oo-ss) and ERp72(ss-ss-oo). Aggregation and ATP secretion of human platelets was potentiated by ERp72(oo-ss-ss) but inhibited by ERp72(ss-oo-ss) and ERp72(ss-ss-oo). These data suggest that both the a and a' active sites are required for platelet function. ERp72 bound poorly to β3-null mouse platelets, and the addition of ERp72(oo-ss-ss) to human platelets generated thiols in αIIbβ3, suggesting a direct interaction of ERp72 with αIIbβ3. Defective aggregation of ERp72-null platelets was recovered by ERp72, but not other thiol isomerases. In summary, ERp72 plays a critical role in platelet function and coagulation through the a and a' CGHC motifs.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis.J Clin Invest. 2015 Nov 3;125(12):4391-406. doi: 10.1172/JCI80319. J Clin Invest. 2015. PMID: 26529254 Free PMC article.
-
A novel role for endoplasmic reticulum protein 46 (ERp46) in platelet function and arterial thrombosis in mice.Blood. 2022 Mar 31;139(13):2050-2065. doi: 10.1182/blood.2021012055. Blood. 2022. PMID: 34752599 Free PMC article.
-
The b' domain of protein disulfide isomerase cooperates with the a and a' domains to functionally interact with platelets.J Thromb Haemost. 2019 Feb;17(2):371-382. doi: 10.1111/jth.14366. Epub 2019 Feb 3. J Thromb Haemost. 2019. PMID: 30566278 Free PMC article.
-
Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis.J Thromb Haemost. 2024 Jul;22(7):1806-1818. doi: 10.1016/j.jtha.2024.03.008. Epub 2024 Mar 20. J Thromb Haemost. 2024. PMID: 38518897 Review.
-
Multiple protein disulfide isomerases support thrombosis.Curr Opin Hematol. 2018 Sep;25(5):395-402. doi: 10.1097/MOH.0000000000000449. Curr Opin Hematol. 2018. PMID: 29994898 Free PMC article. Review.
Cited by
-
Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease.J Biol Chem. 2019 Feb 8;294(6):2133-2141. doi: 10.1074/jbc.TM118.002812. Epub 2018 Dec 12. J Biol Chem. 2019. PMID: 30541925 Free PMC article. Review.
-
Microvascular thrombosis: experimental and clinical implications.Transl Res. 2020 Nov;225:105-130. doi: 10.1016/j.trsl.2020.05.006. Epub 2020 May 23. Transl Res. 2020. PMID: 32454092 Free PMC article. Review.
-
Vascular endothelial ERp72 is involved in the inflammatory response in a rat model of skeletal muscle injury.Mol Med Rep. 2021 Mar;23(3):186. doi: 10.3892/mmr.2021.11825. Epub 2021 Jan 5. Mol Med Rep. 2021. PMID: 33398381 Free PMC article.
-
Oxidative Stress and Preeclampsia-Associated Prothrombotic State.Antioxidants (Basel). 2020 Nov 17;9(11):1139. doi: 10.3390/antiox9111139. Antioxidants (Basel). 2020. PMID: 33212799 Free PMC article. Review.
-
Thioredoxin-related transmembrane protein 1 negatively regulates coagulation and phosphatidylserine exposure.Res Pract Thromb Haemost. 2024 Jun 11;8(4):102472. doi: 10.1016/j.rpth.2024.102472. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 39036672 Free PMC article.
References
-
- Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11(11):2807-2850. - PubMed
-
- Holbrook LM, Watkins NA, Simmonds AD, Jones CI, Ouwehand WH, Gibbins JM. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148(4):627-637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

